Article Text
Abstract
Objectives Th17/IL-17 dysregulation is involved in human autoimmunity, and recent evidence suggests the character of long-lived differentiated memory cells in Th17. By directly measuring the peripheral blood mononuclear cells (PBMC), elevated circulating frequencies of Th17 cells have been reported in systemic lupus erythematosus (SLE) with inconsistent results regarding the correlation with disease activities. In this study, the association between circulating Th17 frequencies and disease activities or laboratory parameters was examined in flow cytometer-sorted CD45RO-positive memory CD4 T cells from SLE.
Methods PBMC samples were obtained from 48 female lupus patients and another 48 age- and sex-matched healthy individuals. We examined frequencies of Th17 cells by sorting the purified CD4 T cells bearing the CD45RO marker, followed by intracellular IL-17A staining after in vitro activation. Frequencies of Th1 and TFoxp3 cells were also measured by intracellular IFN-γ and Foxp3 staining, respectively. The SLE disease activity index (SLEDAI) and other laboratory parameters were further correlated with frequencies of different T cell subsets.
Results In SLE, increased frequencies of Th17 cells were found with a positive correlation in SLEDAI. Higher frequencies of Th17 cells were found in lupus nephritis. There was a positive correlation between frequencies of Th17 cells and daily proteinuria amount.
Conclusions By examining the sorted CD45RO-positive memory CD4 T cells, we confirm the dysregulation of Th17/IL-17 in SLE, implicating the potential to treat lupus patients with selective IL-17/IL-17R blockades.
- Lupus Nephritis
- Systemic Lupus Erythematosus
- T Cells
- Th17
- Flow cytometry sorter
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
CD4 T helper (Th) cells can differentiate from naïve status into antigen-experienced effectors with distinct characteristics, such as the interleukin 17 (IL-17)-secreting Th17 subset. These cells are implicated in autoimmune diseases including rheumatoid arthritis, ankylosing spondylitis, psoriasis/psoriatic arthritis, Sjo˝gren syndrome, multiple sclerosis, inflammatory bowel disease and type 1 diabetes.1 ,2 Patients with systemic lupus erythematosus (SLE) have increased levels of IL-17 in blood and renal tissues, and higher frequencies of Th17 cells are found by directly measuring CD4-positive IL-17-producing T cells upon in vitro activation of peripheral blood mononuclear cells (PBMC), with inconsistent results regarding the correlation between IL-17 levels or frequencies of Th17 cells and disease activities.3 ,4 Indeed, there are difficulties in defining human CD4-positive IL-17-producing cells by flow cytometry because of the very low frequencies in PBMC samples and the problem of background after in vitro activation. Interestingly, higher frequencies of IL-17-producing CCR6/CD45RO-positive memory T cells are found in patients with SLE carrying the type I interferon signature, but there are few published reports characterising the memory cell markers on CD4-positive IL-17-producing T cells.4 ,5
Using PBMC from 48 patients with SLE and 48 age- and sex-matched healthy individuals, we examined the frequency of Th17 cells in purified CD4-positive T cells bearing CD45RO, a well-known differentiated memory T cell marker, followed by intracellular IL-17A staining upon in vitro activation. Th1 and Foxp3-positive T cell subsets were also measured by intracellular interferon γ (IFN-γ) and Foxp3 staining, respectively. In this study we identified increased frequencies of Th17 cells and a positive correlation with disease activity scores and daily amounts of proteinuria in SLE. Higher frequencies of Th17 cells were found in lupus nephritis.
Methods
Patients
Forty-eight women of mean±SD age 38.9±9.6 years (range 21–58 years) fulfilling the American College of Rheumatology revised criteria for SLE were enrolled in the study. Their medical records were reviewed and disease activity was assessed by the SLE Disease Activity Index (SLEDAI). The laboratory parameters analysed included daily amount of proteinuria, anti-DNA levels, C3/C4 concentrations and complete blood cell counts. The diagnosis of lupus nephritis was based on serial examinations of blood and urine samples for the presence of any of the following: (1) a 30% decrease in creatinine clearance in 1 year; (2) 24 h urinary protein >1 g; (3) at least three of the following present in a 1-year period: (a) serum albumin levels <3 g/dL; (b) proteinuria ≥2+; (c) oval fat bodies and granular, hyaline or red blood cell casts in the urine; and (d) persistent haematuria of >5 red blood cells per high-power field.6 Eight patients underwent histological evaluation to provide additional information not obtained in laboratory tests.7 Forty-eight healthy sex- and age-matched individuals (mean±SD age 39.1+9.6 years) served as a control group.
Preparation of CD45RO-positive CD4 T cells
PBMC were isolated from heparinised blood samples by Ficoll-Paque PLUS (GE Healthcare). CD4 T cells were purified by Dynal CD4 Negative Isolation Kit (Invitrogen Dynal AS), stained with FITC-conjugated anti-CD45RO (eBioscience) and isolated by a FACS Aria cell sorter (BD Biosciences) with a purity of >95%.
Calculation of Th17, Th1 and TFoxp3 frequencies
Purified CD45RO-positive CD4 T cells were stimulated with phorbol myristate acetate (5 ng/mL) and ionomycin (500 ng/mL) in the presence of 2 μM monensin (GolgiStop, BD Biosciences) for 4 h, stained with FITC-conjugated anti-CD4 (eBioscience), fixed in 2% paraformaldehyde (Sigma-Aldrich) and permeabilised with 0.1% saponin (Sigma-Aldrich), followed by intracellular staining with PE-conjugated anti-IL-17A or anti-IFN-γ (eBioscience), as described previously.8 For measurement of Foxp3-positive T cells, isolated CD45RO-positive CD4 T cells were stained with FITC-conjugated anti-CD4 (eBioscience), fixed in 2% paraformaldehyde (Sigma-Aldrich), permeabilised with 0.1% saponin (Sigma-Aldrich) and stained with PE-conjugated anti-Foxp3 (eBioscience). Staining by control isotype monoclonal antibodies was included in each sample. The double-stained cells were analysed on a FACSCalibur flow cytometer (BD Biosciences). CD4/IL-17-positive, CD4/IFN-γ-positive and CD4/Foxp3-positive cells were defined as Th17, Th1 and TFoxp3 cells, respectively, and the calculated frequencies were expressed as percentages of the total CD45RO-positive CD4 T cells.
Statistical analysis
Data are presented as mean±SD. The Mann–Whitney rank sum test was used to compare the frequencies of Th17, Th1 or TFoxp3 subsets between patients with SLE and healthy controls, and Pearson correlation coefficient with linear regression analysis was performed to determine the correlation between the frequency of individual subsets and SLEDAI or laboratory parameters. p Values <0.05 were considered to be statistically significant.
Results
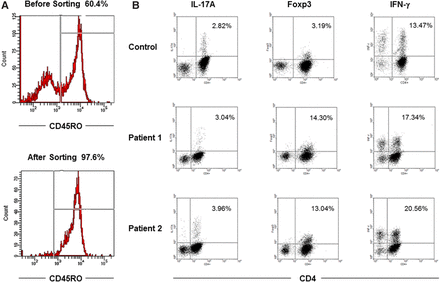
Figure 1A demonstrates a representative picture of sorted CD45RO-positive CD4 T cells with a purity of around 98%. Each subset could be clearly defined by flow cytometric analysis, and three representative pictures are shown in figure 1B.
Representative graphs of flow cytometric analyses. (A) CD45RO purity in isolated CD4 T cells from a patient with systemic lupus erythematosus (SLE) sorted by flow cytometry. (B) Detection of Th17, Treg and Th1 cells from a healthy individual and two patients with SLE.
Patients with SLE had higher frequencies of Th17 cells than healthy subjects (n=48, 3.51±1.32% vs 2.83±1.23%, p=0.0371), as did patients with lupus nephritis (n=24, 3.92±1.53%, p=0.0113) and those with class IV nephritis (n=6, 4.51±1.57%, p=0.0223; figure 2A). There was no difference in the frequency of Th1 cells between patients with SLE and healthy controls (18.47±5.00% vs 19.42±4.67%, p=0.2847), but higher frequencies of TFoxp3 were found in patients with SLE compared with healthy subjects (13.52±4.70% vs 6.77±2.48%, p<0.0001; figure 2B).
Frequencies of Th17, Th1 and TFoxp3 cells in patients with systemic lupus erythematosus (SLE). (A) Increased frequencies of Th17 cells were seen in patients with SLE, lupus nephritis (LN) and class IV nephritis (IV) compared with healthy controls (HC). (B) No difference in the frequency of Th1 cells was seen between patients with SLE and HC, but TFoxp3 cells were increased in patients with SLE in comparison with HC. Horizontal bars in each figure represent mean values.
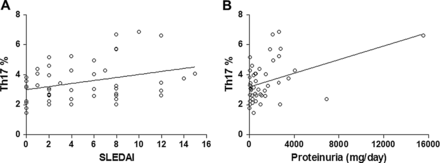
The correlation coefficients between the frequencies of each subset and SLEDAI or laboratory parameters were calculated. There was no statistically significant correlation in the Th1 and TFoxp3 subsets, but a positive correlation with linearity was found between the frequency of Th17 cells and SLEDAI scores (r=0.3228, p=0.0252; figure 3A), and between Th17 cell frequency and amount of proteinuria (r=0.4268, p=0.0025; figure 3B). No correlation was found in other laboratory tests.
Correlation between frequency of Th17 cells and disease activity or amount of proteinuria in patients with systemic lupus erythematosus (SLE). There was a positive correlation with linearity between Th17 frequencies and (A) SLE disease activity index scores (SLEDAI) or (B) amount of proteinuria.
Discussion
In SLE there is persistent activation of the immune system with production of cytokines, and elucidation of this mechanism would contribute to the development of novel therapeutics targeting pathogenic cytokines, their receptors or components of the related signalling pathways.4 Recently, biologics targeting IL-17A or IL-17RA have successfully entered clinical trials.2 Using lupus mouse models, increased levels of IL-17 and an increase in the number of Th17 cells have been identified in renal tissue, and abrogating the IL-17R signalling pathway prevents the development of nephritis.3 ,4 Since there is a high prevalence of SLE with widespread usage of biologics in this area, establishing Th17/IL-17 immune dysfunction could provide solid evidence for initiating clinical trials in patients with selective IL-17/IL-17R inhibitors.9
Memory T cells are the most abundant lymphocyte population undergoing progressive differentiation from activated naïve status upon antigen exposure to circulating subsets with different surface markers, and increasing evidence suggests that human Th17 cells resemble long-lived differentiated memory T cells.10 ,11 Interestingly, patients with SLE have lower circulating frequencies of CD45RO-negative naïve CD4 T cells.12 By directly measuring PBMC, increased frequencies of Th17 cells have been reported in SLE, but there is controversy over the correlation of Th17 status with disease activity and characterisation of the memory T cell markers is restricted.3 ,4 In this study we were able to detect the Th17 population by using sorted high-purity CD45RO-positive CD4 T cells, and our findings clearly demonstrate increased Th17 frequencies with a positive correlation with disease activity and with the amount of proteinuria in patients with SLE. Nevertheless, only 8 of the 24 patients (33.3%) with lupus nephritis were confirmed by histopathological examination, which limits the clinical significance of this study. Further research to analyse patients with different histopathological classes may provide a better insight into the pathogenic mechanisms of the CD45RO-positive Th17 population in lupus nephritis.
The development pathways of Treg and Th17 cells are reciprocally regulated with the influence on the outcome of pathological immune responses.13 Notably, Treg cells lose their suppressive function and acquire the effector phenotype under certain inflammatory milieu, suggesting instability and plasticity of this population.14 In contrast to reciprocal changes, with an inverse association between Th17 and Treg subsets in PBMC from SLE,4 concomitantly increased frequencies of Th17 and TFoxp3 cells in CD45RO-positive CD4 memory T cells with a positive correlation (r=0.4348, p=0.0020) were identified in this study. In fact, many aspects of the Th17/Treg dysregulation in the disease course of SLE remain uncertain with no conclusive pathogenesis, and contradictory data regarding the number and function of peripheral Treg cells in human autoimmunity is still a subject for debate.15 Further studies analysing miscellaneous markers of Treg cells and examination of their transformation mechanisms into effector phenotypes might clarify these issues.
In conclusion, by examining sorted CD45RO-positive memory CD4 T cells, increased circulating frequencies of Th17 cells were found in parallel with disease activity and the amount of proteinuria in patients with SLE. We confirm the dysregulation of Th17/IL-17 in SLE, implicating a therapeutic potential for the treatment of lupus patients with selective IL-17/IL-17R blockade.
Acknowledgments
The authors are indebted to Ms Fang-Lin Chiu for her technical assistance with the laboratory work.
References
Footnotes
-
Funding This study was supported by grants NSC-100-2314-B-006-033, NSC-101-2314-B-006-042-MY3 and MOST 103-2314-B-006-058-MY3 from the Ministry of Science and Technology, Republic of China.
-
Contributors M-FL designed the study; M-FL and C-RW collected and interpreted the experimental data; C-RW wrote the manuscript.
-
Competing interests None.
-
Patient consent Obtained.
-
Ethics approval The Institutional Review Board of National Cheng Kung University Hospital approved this study.
-
Provenance and peer review Not commissioned; externally peer reviewed.