Article Text
Abstract
Objective Systemic lupus erythematosus (SLE) is associated with increased risk of adverse pregnancy outcomes, including pre-eclampsia, particularly in association with antiphospholipid antibody syndrome (APS). While significant placental abnormalities are expected in pre-eclampsia, less is known about how lupus activity and APS in pregnancy affect the placenta. We describe placental pathology from a population of lupus pregnancies, several of which were complicated by APS-related thromboses, in which pre-eclampsia and other complications developed. We performed standard histopathological placental review and quantified neutrophils and neutrophil extracellular traps (NETs) in the intervillous space, given the recognised association of NETs with lupus, APS and pre-eclampsia.
Methods Pre-eclampsia, SLE and control placentas were scored for histological features, and neutrophils were quantified on H&E and immunohistochemical staining for the granular protein myeloperoxidase. NETs were identified by extracellular myeloperoxidase staining in the setting of decondensed nuclei. Non-parametric analysis was used to evaluate differences in netting and intact neutrophils between groups, with Kruskal–Wallis testing for associations between histological findings and neutrophils.
Results Placentas were evaluated from 35 pregnancies: 10 controls, 11 pre-eclampsia, 4 SLE+pre-eclampsia and 10 SLE, including one complicated by catastrophic APS and one complicated by hepatic and splenic vein thromboses during pregnancy. Intrauterine growth restriction and oligohydramnios were observed in lupus cases but not controls. Significantly more NETs were found infiltrating placental intervillous spaces in pre-eclampsia, SLE+pre-eclampsia and all 10 SLE non-pre-eclampsia cases. The ratio of NETs to total neutrophils was significantly increased in all case groups compared with controls. When present, NETs were associated with maternal vasculitis, laminar decidual necrosis, maternal–fetal interface haemorrhage and non-occlusive fetal thrombotic vasculopathy.
Conclusions In this pilot study of placental tissue from lupus pregnancies, outcomes were more complicated, particularly if associated with APS. Placental tissue revealed marked inflammatory and vascular changes that were essentially indistinguishable from placental tissue of pre-eclampsia pregnancies.
- Systemic Lupus Erythematosus
- Autoimmune Diseases
- pregnancy
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Pregnancy management in systemic lupus erythematosus (SLE) has improved over years, with more emphasis on multidisciplinary management and continuation of antimalarial therapy in pregnancy leading to increasingly better outcomes.1 While recent evidence suggests that lupus pregnancies, particular those with low disease activity, have better outcomes than previously thought, SLE pregnancies remain at significantly increased risk for complications, including pre-eclampsia, intrauterine growth restriction (IUGR), prematurity and maternal and fetal mortality, particularly if disease activity is high during pregnancy or if antiphospholipid antibodies are present.2–4 Because it can present similarly to proliferative lupus nephritis, pre-eclampsia, a hypertensive disease of pregnancy, can be one of the most difficult complications to diagnose and treat in pregnant patients with lupus. Pre-eclampsia is characterised by new hypertension and proteinuria after the 20th week of gestation, occurring in about 2–8% of pregnancies in general,4 and a full 22.5% of lupus pregnancies. Both lupus and pre-eclampsia are characterised by similar histopathological placental pathology indicative of underlying derangements in implantation, vascular remodelling, and immune regulation.4 ,5 These similarities raise the possibility that a common pathogenesis may exist along a spectrum in both lupus and pre-eclampsia pregnancies, sharing joint mechanisms and outcomes.
A recently proposed mechanism for the increased endothelial dysfunction, thrombosis and perpetuation of the inflammatory milieu observed in SLE involves the finding of increased neutrophil extracellular traps (NETs) in the circulation, skin and renal tissues of patients with lupus.6 ,7 Neutrophils form NETs by releasing a web of nuclear contents including chromatin, DNA and antimicrobial proteins such as neutrophil elastase, myeloperoxidase (MPO), cathepsin G and proteinase 3 into the extracellular space in response to microbial triggers including bacteria, viruses and fungi.8 As such, NET release (or NETosis) represents an important arm of antimicrobial innate immunity, and one distinctly different from the process of phagocytosis, which typically leads to neutrophil apoptosis.9 ,10 In addition to the response to microbes, NET release is triggered by various stimuli, including inflammatory cytokines,11 ,12 immune complexes13 and placental syncytiotrophoblast microparticles found in the maternal circulation in pre-eclampsia.12 Upon release, NETs mediate toxic effects on other bystander cells, including vascular endothelial cells,14 and exert a powerful prothrombotic effect.15 ,16
Interestingly, a propensity of neutrophils to form NETs has been observed in patients with SLE, in whom both an impaired ability to degrade NETs and a predilection of neutrophils to undergo NETosis has been observed.14 ,17–19 NETs may therefore have a prominent role in inducing and perpetuating the vasculopathy observed in SLE, as well as other autoimmune diseases such as antineutrophil cytoplasmic antibody-associated vasculitis, antiphospholipid antibody syndrome (APS) and possibly pre-eclampsia, where previous research has identified NETs infiltrating the placental intervillous space in pregnancies complicated by pre-eclampsia.16 ,20 ,21
To test the hypothesis that the presence of neutrophils and NETs in the placental intervillous space are common features of pregnancies in both lupus and pre-eclampsia, and that their presence corresponds to ongoing inflammatory and vasculopathic changes throughout the placenta, we examined histological features of placental tissue from women who had lupus or pre-eclampsia (or both) complicating their pregnancies, and compared them with placental tissue from normal pregnancies. In addition to scoring the extent of tissue inflammation, maternal–fetal interface haemorrhage and infarction in these samples, we quantified intact and netting neutrophils detected by immunohistochemical staining, focusing on the placental intervillous space, the site of oxygen and nutrient exchange. We hypothesised that both lupus pregnancies and pre-eclampsia would be associated with inflammatory histological features, as well as the infiltration of neutrophils/NETs, compared with normal pregnancies. We expected to see an overall more benign histology and fewer total neutrophils and NETs in the lupus pregnancies than in the pre-eclampsia group, in whom abnormal placental pathology would be expected.
Materials and methods
Approval for this research was obtained through the University of Michigan Institutional Review Board.
Case ascertainment
SLE and pre-eclampsia cases were identified using corresponding ICD-9 codes at admission and discharge (see online supplementary appendix 1 for list of codes). A cross-reference of SLE and pre-eclampsia cases was then performed to capture lupus pregnancies complicated by pre-eclampsia. From each group, social history was reviewed, and cases with positive tobacco use during pregnancy were excluded. Cases with pre-existing or gestational hypertension and diabetes were excluded from the study. Multifetal gestations and fetuses with structural/chromosomal anomalies were excluded.
Supplemental material
Verification of SLE cases
Charts review was performed for subjects identified by ICD-9 search in order to confirm SLE diagnosis by ≥4/11 American College of Rheumatology classification criteria.22 ,23 Data were obtained from inpatient and outpatient records, including scanned documents within subject files from outside rheumatologists where, in some cases, the lupus diagnosis was made prior to receiving care at the University of Michigan. Other data reviewed included laboratory results with particular attention to autoantibody profile, haematological and renal testing, as well as pathology review of renal biopsies. Assessment of SLE disease activity during pregnancy was based on the treating rheumatologists' assessment and on whether an increase in the patient's daily prednisone dose of ≥10 mg/day was made at any time during pregnancy. All but one patient with SLE were evaluated and treated by University of Michigan rheumatologists during their pregnancies. The one patient treated outside the University of Michigan system had inactive lupus during pregnancy based on obstetric notes, concurrent medications and laboratory measures.
Forty-three per cent of patients with SLE in the study had antiphospholipid antibodies, categorised as follows: IgG and IgM isotypes of anticardiolipin (aCL) and β-2-glycoprotein 1 (β-2GP1) were considered positive if greater than the 99th percentile at our institution, on two or more occasions, at least 12 weeks apart, 6 months prior to or during the study case pregnancy. This corresponded to the following values: aCL IgG (≥22 GPL); aCL IgM (≥25 MPL); β2GP1 IgG (≥24 U/mL); β2GP1 IgM (≥26 U/mL). The lupus anticoagulant was identified either by prolongation of activated partial thromboplastin time or dilute Russell's viper-venom time with a positive confirmatory test. 28.5% of patients with SLE fulfilled diagnostic criteria for APS according to expert consensus criteria,24 ,25 which is independently associated with pregnancy complications.26 For purposes of analysis, patients with secondary APS were included within the ‘SLE’ group.
Verification of pre-eclampsia cases
Chart review was performed for subjects identified by ICD-9 search in order to confirm pre-eclampsia diagnosis occurring in both the antepartum and postpartum periods. Pre-eclampsia is defined by the International Society for the Study of Hypertension in pregnancy as hypertension of at least 140/90 mm Hg on two separate occasions ≥4 h apart, in addition to proteinuria of at least 0.3 g/24 h collection (or >30 mg/mmol protein/creatinine ratio), occurring after the 20th week of gestation in previously normotensive women, with resolution by the 6th week of the postpartum period.4 ,27 This diagnosis was met in 9/11 of the subjects; the diagnosis in the remainder of cases was based on the clinical judgement of the treating obstetrician in the context of hypertensive emergency and proteinuria as they were delivered emergently.
Control cases
A prospective control group of sequentially enrolled healthy women undergoing planned caesarean-sections between 38 and 39 weeks gestation was identified, and placental tissue obtained at the time of delivery. One patient delivered spontaneously at the time of her planned section.
Tissue procurement and processing
Formalin-fixed, paraffin-embedded tissue was obtained from the University of Michigan Pathology Slide/Block Library Repository. Placental tissue from women who deliver at the University of Michigan Hospital is saved and fixed in 10% formalin solution when a pathologist's review is requested by the obstetrician or perinatologist. In the majority of SLE pregnancies (approximately ≥90%) at our hospital, a formal placental pathology review is performed due to recognition of higher risk and adverse outcomes.
Review of pathology
All slides were reviewed with an experienced placental pathologist (RWL), who was blinded to the clinical outcome.
Histological review
All specimens were first characterised by H&E staining. We used standard diagnostic criteria and performed a systematic pathologic review adapted from work by Salafia and Popek, developed with the intention of identifying underlying pathophysiological processes with the most relevant clinicopathological correlations.28 ,29 These factors included but were not limited to acute and chronic inflammation, vasculopathy, maternal–fetal interface haemorrhage and ischaemic changes detailed below and described in online supplementary table (appendix 2). The separate histological categories were scored as follows: ‘absent=0; mild=1; moderate=2; severe=3’ for categorical variables, or ‘present/not present’ for binary variables. The relationship between these variables and the presence of neutrophils and NETs in the intervillous space was then assessed.
Acute and chronic inflammation
An acute maternal inflammatory response was considered mild if neutrophils were present, including amnionitis, focal deciduitis or intervillositis; moderate if more global inflammatory changes and obvious neutrophils were apparent on low power; or severe if microabscess formation and coalescing neutrophils were seen. An acute fetal inflammatory response was mild if focal vasculitis was seen; moderate with acute fetal inflammation (funisitis, which is defined by the finding of polymorphic mononuclear cells in the umbilical cord vasculature and stroma), of at least one vessel; or severe if funisitis was present. Chronic decidual inflammation was considered mild if a non-occlusive perivascular infiltrate was noted with intramuscular histiocytes and vascular degeneration; moderate with multifocal, partially occlusive thrombosis; or severe with thrombosed vessels and palisading inflammatory in the vessel wall, with or without fibrinoid necrosis.
Vasculopathy and infarction
Histological changes of vasculopathy of the maternal and fetal circulations were qualitatively assessed. Fetal thrombotic vasculopathy was assigned to distal vessel and large vessel involvement and scored based upon degree of involvement. Villous hypervascularity as documented by the presence or absence of chorangiosis, which is defined by at least 10 villous capillaries in 10 villi on a 10× objective, seen in multiple locations, was also determined. Maternal decidual vasculopathy was scored as mild to moderate depending on the presence and extent of atherosis and/or perivascular alteration of spiral arterioles, and severe if multiple vascular lumens were replaced by organised thrombosis and fibrinoid necrosis of vessel walls.
Maternal–fetal interface haemorrhage
The presence of acute and chronic maternal–fetal interface haemorrhage was also assessed as it implies an implantation site defect, which has been traditionally associated with pre-eclamptic pregnancies. Due to the small sample size, acute and chronic haemorrhage were considered as one group.
Neutrophil quantification
Using an ocular micrometer, the diameter of a 60× high-power field was measured as 0.36 mm (360 µm). Neutrophils were identified by MPO immunohistochemistry (as described below), as well as by nuclear morphology. Placental sections were evaluated at 60×, with neutrophil counts obtained in 10 consecutive fields in which the maternal intervillous space was identified. A neutrophil was defined as ‘intact if MPO staining was confined within the cytoplasm of the cell, and if an intact, multilobulated nucleus was present. A neutrophil was defined as ‘netting’ when extracellular MPO was detected in the presence of a disrupted, decondensed nucleus. Finally, neutrophils identified as ‘indeterminate’ when extracellular MPO was present, but with unclear nuclear-cytoplasmic integrity. These cells were counted as part of the total number of neutrophils in the intervillous space but were not considered to be forming NETs.
Immunohistochemistry
Deparaffinisation and rehydration were performed with standard xylene-to-ethanol washes. Heat-induced epitope retrieval was achieved by boiling samples for 30 min in sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0). Samples were blocked in phosphate buffered saline plus 0.025% Tween 20 with 10% foetal bovine serum. The primary antibody was to MPO (Dako, Carpinteria, California, USA), while the secondary antibody was HRP-conjugated anti-rabbit IgG (Amersham Biosciences, Pittsburgh, Pennsylvania, USA). In between the primary and secondary antibodies, tissue was incubated for 15 min in 0.3% hydrogen peroxide to block endogenous peroxidase activity. Colour change was detected with the DAB-Plus Substrate Kit (Invitrogen, Grand Island, New York, USA). Samples were counterstained with haematoxylin. For a subset of the samples, the identity of netting neutrophils was confirmed (as MPO-DNA overlap) by immunofluorescence staining with anti-MPO (Dako) and DAPI as previously described.30
Statistical analysis
Summary statistics were computed to describe the distributions of variables of interest, and boxplots stratified by patient group were used for graphical display. Logistic regression was used to model the associations between number of netting neutrophils and binary histopathological outcome measures. Due to the sample size and data restriction, the non-parametric Kruskal–Wallis test was used to evaluate whether the ratio of netting neutrophils to intact neutrophils differed between groups.
Results
Demographics
Placentas from 10 healthy controls, all of whom were scheduled for planned delivery by caesarean-section were compared with placentas from 10 SLE, 11 pre-eclampsia and 4 SLE+pre-eclampsia cases. SLE cases from deliveries occurring during years 2004–2011 were included. Detailed demographic and clinical data are presented in table 1.
Characteristics of study population
The maternal ages at the time of delivery were comparable between controls and patients with SLE (35.5 (SD±4.12) and 31.7 (SD±5.93), respectively), whereas patients with pre-eclampsia were younger at age of delivery (29 (SD±4.98)). Not surprisingly, all three case groups had significantly shorter mean gestational periods than controls (control vs pre-eclampsia, p=0.034; control vs SLE, p=0.0019; control vs SLE+pre-eclampsia, p=0.0105). The indications for delivery among non-pre-eclampsia lupus subjects were as follows: oligohydramnios (three); premature rupture of membranes (two); IUGR (two), one in a mother with hepatic and splenic thromboses due to APS; breech presentation (one); and increasing MCA Doppler (concern for fetal cerebral oedema) (one). All SLE cases had positive antinuclear antibody (by HEp-2 immunofluorescence). A remote history of biopsy-proven lupus nephritis was present in two in the SLE group and one of the SLE+pre-eclampsia group, but none had baseline renal impairment. Among SLE cases, two developed severe third trimester lupus/APS complications: one developed lupus nephritis and subsequent catastrophic APS in the third trimester, and one developed hepatic and splenic infarction due to APS. One woman experienced a third trimester lupus flare based on malar rash and inflammatory arthritis, with hypertension and proteinuria. She was treated with moderate-dose steroids for lupus and delivered urgently out of concern for pre-eclampsia, which was confirmed by subsequent prompt resolution of hypertension and proteinuria. Among the remainder of SLE cases, disease control was achieved during pregnancy, and no adjustments of prednisone >10 mg/day were made with the exception of dexamethasone administered for the indication of congenital heart block (in two anti-Ro/SSA, anti-La/SSB antibody-positive SLE cases). No cases of chorioamnionitis were identified. One case was excluded from analysis due to prolonged rupture of membranes (eg, lasting longer than 18–24 h between time of rupture and time of delivery),31 and concern for NET induction in response to ascending bacterial infection.
Numbers of neutrophils and neutrophil NETS, and the ratio of NETs/total neutrophils are significantly higher in intervillous space of SLE and pre-eclampsia than controls
Representative histological examples of intact and netting MPO-stained neutrophils in placental intervillous space are shown in figure 1. Neutrophils with clearly granular MPO staining are representative of intact neutrophils (figure 1A). Neutrophils in which there is a disrupted, decondensed nucleus, with MPO staining, extracellularly represent netting neutrophils (figure 1B). As further evidence of the netting process, a representative image of immunofluorescence staining with anti-MPO and DAPI (for DNA) for two SLE cases is demonstrated in figure 2.
Myeloperoxidase staining of the intervillous space from a healthy control pregnancy (A), a lupus pregnancy (B). Intact neutrophils (black arrows) and netting or non-intact neutrophils (white arrows) are identified.
Netting neutrophils were confirmed (as myeloperoxidase (MPO)-DNA overlap) by immunofluorescence staining with anti-MPO (green) and DAPI (DNA=blue). These are representative images from two separate patients with systemic lupus erythematosus. The left image shows two discrete neutrophil extracellular traps, while the right image highlights an area of widespread netting. Scale bar=100 μm.
The data for neutrophil and NET quantification in 10 consecutive fields at 60× high-power field are presented in table 2.
Neutrophil and neutrophil extracellular trap (NET) quantification (median (IQR)) in the placental intervillous space
Of note, scarce, if any, NETs were seen in the controls. The numbers of both total neutrophils and neutrophil NETs in placental intervillous space were significantly higher in the pre-eclampsia and SLE groups compared with controls. These values were also higher in the SLE+pre-eclampsia group compared with controls, though not reaching statistical significance, likely due to the small sample size in this group (n=4). Among the case groups (pre-eclampsia, SLE and SLE+pre-eclampsia), the counts of total neutrophils and NETs did not differ statistically.
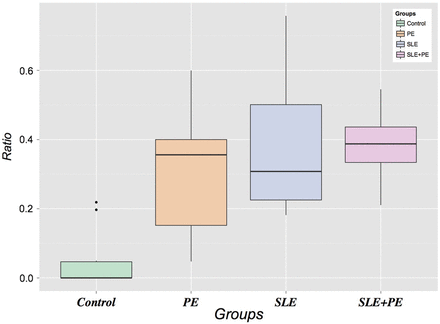
Furthermore, we also assessed the relative proportions of neutrophils forming NETs within each group by calculating the ratio of NETs to total neutrophils (intact, indeterminate and netting). As displayed in figure 3, the NET:total neutrophil ratios were significantly higher in each of the three case groups compared with controls, demonstrating a higher level of NETosis accounting for the absolute neutrophil numbers. The magnitude of the NET:neutrophil ratio was highest in the combined SLE+pre-eclampsia group. There was no correlation between neutrophils or NETs and the duration of membrane rupture, making ascending infection as a cause of neutrophil infiltration less likely. In summary, these data suggest that the large neutrophilic infiltrate in the intervillous space of SLE and pre-eclampsia pregnancies is a prominent and common feature of both processes that is not present in normal pregnancies.
Ratio of neutrophil extracellular traps to total neutrophils (intact, indeterminate and netting). SLE, systemic lupus erythematosus.
NETs predict the presence of histopathological findings
We examined histopathological features on H&E-stained placental tissue with particular attention to vascular and inflammatory phenomena for correlation with the presence of NETs in the intervillous space (table 3).
The presence of intervillous neutrophil extracellular traps (NETs) versus no NETs in all cases corresponds to vascular pathology and inflammation*
Of the histopathological features, the presence of NETs in the intervillous space corresponded primarily to pathologic vascular and inflammatory changes of maternal decidua. In particular, NETs were associated with laminar necrosis of placental membranes, and maternal–fetal interface haemorrhage. NETs also correlated to the presence of fetal non-occlusive thrombotic vasculopathy. However, we did not observe a correlation with NETs and more extensive fetal vascular pathology: for example, there was no association with the presence of atherosis, distal villous or large vessel lesions, or villous infarct, although that could be due in part to limited sample size. Acute maternal inflammation as a group (intervillositis, amnionitis) neared a significant association with the presence of NETs in the intervillous space; however, chronic inflammatory changes did not.
Discussion
In this study, we performed a detailed pathological review of placentas from lupus pregnancies and from pre-eclampsia pregnancies. The lupus pregnancies occurred between years 2004 and 2011, during which time improvements in management of lupus pregnancy were occurring, due to multidisciplinary approaches in care including preconception counselling, a general acknowledgement that 6 months of good lupus disease control prior to conception improved outcomes and the continuation of antimalarial therapy throughout pregnancy.32 The placental pathology from lupus pregnancies exhibited features of inflammatory vasculopathy comparable to pre-eclampsia, arguably the most severe and potentially fatal disease of pregnancy. In particular, a more robust non-infectious infiltrate of both intact neutrophils and NETs in the placental intervillous space was present in both lupus and pre-eclampsia compared with control pregnancies. Further, these infiltrates corresponded to histological changes of the placenta consistent with inflammation and vasculopathy (decidual vasculitis, laminar necrosis, maternal–fetal interface haemorrhage, fetal thrombotic vasculopathy). These findings are intriguing in the context of the well-recognised toxic effect of NETs on vascular endothelium and raise the possibility that NETs are actually driving the observed histopathological changes.
These abnormal placental findings may represent another manifestation of subclinical vascular disease that is well described in SLE, both in studies of carotid intimal media thickness33–35 and abnormal myocardial perfusion.36 Indeed, increased risk of cardiovascular disease in both lupus and in pre-eclampsia, in the absence of traditional Framingham risk factors, is recognised to contribute to the excess morbidity and mortality observed in long-term follow-up of these populations.37–41 Our findings suggest a similar phenomenon occurring within the placenta, a highly vascularised target organ, corresponding to the observed high rates of IUGR and oligohydramnios in these pregnancies. IUGR in turn has been observed at a rate of 5.6% in lupus pregnancies from the Nationwide Inpatient Sample, as opposed to 0.09% in the general population,2 and in 20% of the small lupus population from our study.
In SLE vasculopathy, the imbalance between vascular damage and repair is characterised in part by increased endothelial cell apoptosis and decreased number and function of endothelial progenitor cells.35 A similar phenomenon has been observed in the placentas of patients with pre-eclampsia, in which increased trophoblast apoptosis and impaired spiral artery structure and function are observed.42 In SLE, increased endothelial cell apoptosis is associated with a novel proinflammatory subset of lupus neutrophils, the ‘low-density granulocyte’ (LDG), which expresses significantly higher levels of type I interferon mRNA compared with control or autologous lupus neutrophils, and is especially predisposed to NET formation.14 ,43 ,44 The same NET contents that disarm and kill bacteria extracellularly are toxic to endothelial cells in vitro14 and may have similar toxic or ‘pro-apoptotic’ effects on trophoblasts, resulting in impaired placentation.
NET formation may also result in externalisation of matrix metalloproteinases (MMPs), which are also present within neutrophil granules.45 MMPs, a family of proteases that degrade extracellular matrix proteins, can damage vascular cell endothelial integrity and function.46 Higher levels of MMP-9 in particular are externalised by NETs formed from lupus LDGs, which, after activation with MMP-2, have been shown to impair aortic endothelium-dependent vasorelaxation and induce endothelial cell apoptosis in lupus-prone mice.45 The imbalance between MMPs and their inhibitors may also impact uterine spiral artery remodelling,47 thereby adversely affecting placental implantation, growth and development in both SLE and pre-eclampsia.
A causal role for inflammation in the genesis of pre-eclampsia has not been established. However, a 2005 paper by Gupta et al12 described NETs infiltrating the intervillous space of placentas from patients with pre-eclampsia, with proposed neutrophil activation by syncytiotrophoblast microparticles as the triggering event for NET formation. Here, using different methods to identify neutrophil NETs, we observed NETs in the intervillous space of patients with pre-eclampsia and for the first time in the intervillous space of patients with SLE. In this setting, NETs may provide a source of immunostimulatory, pro-inflammatory and antiangiogenic mediators that are passed on to the fetus with unknown consequences.
Study limitations include the lack of prospectively obtained lupus disease assessment using a validated activity instrument (eg, the SLE Disease Activity Index):48 rather, assessment of lupus activity was based on chart review by a study rheumatologist with experience in lupus (WM) with attention to medication changes, including prednisone adjustments. Additionally, the number of cases, particularly among the SLE+pre-eclampsia group, was small: a larger sample size could adequately assess whether the combination of both processes results in a more aggressive immunological process. Finally, it is difficult to ascertain whether the pathological findings observed in this placental tissue represent cause or effect of observed birth outcomes such as oligohydramnios or IUGR, and to what extent underlying SLE and APS contribute.
The strengths of this study include a rigorous histopathological review and neutrophil quantification of all placental tissue by an experienced placental pathologist, as well as novel immunohistochemical staining for neutrophil NETs in an experienced lab with a focus on NET biology. The findings of this novel work add to a growing body of research related to lupus pregnancy outcomes. Over the past several decades, increasingly more women with SLE are achieving successful pregnancies thanks to improvements in disease diagnosis and management.49–51 Indeed, recent findings from the PROMISSE study reveal better outcomes of lupus pregnancies than had been previously observed.52 The findings from the current study, revealing a higher rate of adverse pregnancy outcomes and abnormal placental structure and function among this group of generally well-controlled lupus pregnancies, raise the issue of long-term implications for the developing fetus. Our findings support observations that lupus pregnancies, particularly in association with antiphospholipid antibodies, are more at risk for complications, even with good disease control. Future directions for this work should focus on characterising the role of neutrophils and NETs on placental development and function in lupus pregnancies as well as focusing on associations between placental pathology observed in this study with long-term developmental outcomes of lupus offspring.
References
Footnotes
Contributors All authors involved in this paper contributed substantially to the conception, design or conduction of the research or the acquisition, analysis or interpretation of data for the work. Additionally, all authors were involved in drafting the work or revising it critically for important intellectual content. Furthermore, all authors gave final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding WM was supported by K12HD001438 from NIH. ECS was supported by NIH 5K01ES019909. JSK was supported by NIH K08AR066569 and a career development award from the Burroughs Wellcome Fund. This work was performed while MJK was employed at the University of Michigan.
Disclaimer The opinions expressed in this article are the author's own and do not reflect the views of the National Institutes of Health, the Department of Health and Human Services, or the US government.
Competing interests None declared.
Ethics approval University of Michigan Medical School Internal Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.