Article Text
Abstract
Background Some studies suggest that the risk for and severity of systemic lupus erythematosus (SLE) can be modified by certain nutrients. The aim of this study was to investigate the association between diet and glucocorticoid (GC) treatment, as a proxy for disease activity, in patients with SLE.
Methods We included 111 patients with SLE from the SLE Vascular Impact Cohort (SLEVIC). Dietary data were linked with data on GC treatment during a 2-year period. The association between diet and GC treatment was analysed with logistic regression. GC treatment and unchanged/increased doses were considered a proxy for active SLE.
Results During the 2-year period, 54 patients (48.6%) had continued GC treatment. Dietary vitamin D was associated with GC treatment (OR=2.70–2.85 (95% CI 1.00 to 8.11)), whereas alcohol was inversely associated with GC treatment (OR=0.28–0.39 (95% CI 0.10 to 98)). Beta-carotene, fatty acid C18:2 and vitamin B6 were inversely associated with unchanged/increased GC dose (OR=0.29–0.30 (95% CI 0.10 to 0.90)). Finally, total energy intake was associated with GC doses >5.0 mg/day and >7.5 mg/day, explaining a direct association between 35 nutrients and higher GC dose levels (OR=2.98–23.82 (95% CI 1.01 to 203.88)).
Discussion Dietary vitamin D did not protect against lupus activity. Beta-carotene, fatty acid C18:2 and vitamin B6 may protect against increased GC dose. The inverse association between alcohol intake and GC treatment/lupus activity may provide a partial explanation for the link between moderate alcohol intake and reduced risk of SLE. The association between higher dietary intake and higher GC dose levels indicated GC's influence on increasing appetite.
- Systemic Lupus Erythematosus
- Corticosteroids
- Disease Activity
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
Vitamin D did not protect against lupus activity.
Beta-carotene, fatty acid C18:2 and vitamin B6 may protect against increases in GC dose.
Higher GC dose levels indicated increased appetite.
Introduction
Glucocorticoids (GCs) are very effective in treating inflammation, but long-term GC treatment is associated with several side effects.1 ,2 GC treatment may reflect disease activity in patients with systemic lupus erythematosus (SLE) with regard to GC treatment dose change over time and/or dose levels. Patients with higher disease activity might be more dependent on GCs than those with lower disease activity. In addition, higher GC doses may be seen in patients with higher disease activity.3 ,4
Little is known regarding the dietary habits in SLE. A few studies have reported that patients with SLE might have a poor nutritional status compared with the general population, showing low consumption of fruits and vegetables as well as inadequate dietary intake of fibre, vitamin B6, calcium and polyunsaturated fatty acids, especially omega-3 fatty acids.5–7 Elkan et al8 have, in the present cohort, specifically found decreased dietary intake of polyunsaturated fatty acids (including omega-3 and omega-6) and fibre compared with healthy controls. Previous findings suggest that vitamin D and moderate alcohol intake are beneficial for patients with SLE. Vitamin D deficiency has been linked with higher disease activity in patients with SLE9–12 and moderate alcohol consumption has shown to be associated with reduced risk of SLE.13–15 However, there is a lack of studies on the link between diet and treatment results in SLE. Since GCs are frequently used to control active SLE, our interest was to focus on the association between diet and GC treatment. The aim of this study was to investigate whether diet influences GC treatment in patients with SLE.
Methods
Study participants
This study included patients with SLE, fulfilling the 1982 revised criteria of the American College of Rheumatology (ACR) for SLE, from the SLE Vascular Impact Cohort (SLEVIC), Karolinska University Hospital, Stockholm, Sweden. Recruitment and inclusion for SLEVIC have been previously described in detail by Anania et al.16 The inclusion criterion was all patients who completed Food Frequency Questionnaire (FFQ) at inclusion.
Dietary assessment
Patients were asked to complete a semiquantitative FFQ at inclusion. This self-reported FFQ involved frequency of intake of 88 food items and beverages during the previous year from inclusion. Completed FFQs were evaluated and an estimation of daily mean intake of 49 nutrients was calculated, using nutrient composition values based on the Swedish National Food Administration data.
GC treatment
Data on GC use and dose levels were extracted from medical records at three time points; at inclusion and at 1 year before and after inclusion. Based on GC use at the three time points, GC dose changes over time, reflecting disease activity, were adjusted for three time periods: previous year to inclusion, following year from inclusion and 1 year before to 1 year after inclusion. GC use over time was categorised into four treatment status groups: ‘none’, ‘discontinued’, ‘started’ and ‘continued’. Dose changes over time were categorised into ‘decreased’, ‘unchanged’ and ‘increased’. GC treatment and higher dose levels (>5.0 mg/day and >7.5 mg/day) were considered a proxy for more active SLE; unchanged or increased GC doses were considered as unfavourable outcomes.
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics V.22. We linked dietary data from FFQ with data on GC treatment from medical records for the year preceding and the year following the FFQ. Associations between diet and GC treatment during the 2-year period were analysed with logistic regression adjusted for age and gender. All nutrient variables were dichotomised into lower and higher intakes with a cut-off at median levels.
Results
This study included 111 patients with SLE. Patient characteristics are presented in table 1.
Patient characteristics at inclusion
During the 2-year period, the percentage of patients treated with GC ranged between 56.8% and 59.5%. In addition to GC, the most common treatments were hydroxychloroquine (HCQ) and azathioprine (AZA); the percentages of patients treated with HCQ and AZA ranged between 40.5–46.8% and 18.9–25.2%, respectively (table 2). Almost all the patients who were treated with GC at inclusion took also supplementation of calcium+vitamin D (66.7%) or calcium (24.2%) or vitamin D (6.1%) alone.
Overview of treatment use and doses at −1 year, inclusion and +1 year
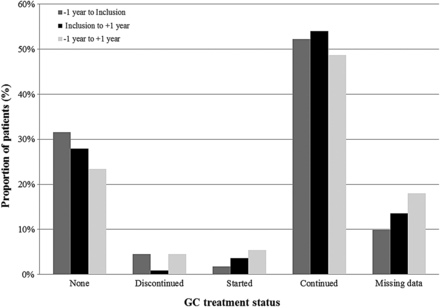
During the 2-year period, 26 patients (23.4%) did not use GC, 4.5% discontinued their GC treatment, 5.4% started GC treatment and 48.6% had continuous GC treatment. 20 patients (18%) had missing data. The distribution of GC treatment statuses over the three time periods is presented in figure 1.
Treatment status of glucocorticoid (GC) from −1 year to inclusion, inclusion to +1 year and –1 year to +1 year.
Of the 54 patients who had continued treatment over the 2-year period, 31.5% had decreased dose, 35.2% had unchanged dose and 33.3% had increased dose. GC dose changes during the three time periods are presented in table 3.
GC dose change (decreased/unchanged/increased) from –1 year to inclusion, inclusion to +1 year and –1 year to +1 year
The association between dietary nutrient intake and GC use is presented in table 4. Vitamin D was associated with GC treatment (OR 2.70–2.85, (95% CI 1.00 to 8.11)), whereas alcohol was inversely associated with GC treatment (OR 0.28–0.39, (95% CI 0.10 to 98)).
The association between dietary nutrient intake and GC use between −1 year to inclusion, inclusion to +1 year and −1 year to +1 year
The association between dietary nutrient intake and unchanged/increased GC dose is presented in table 5. Beta-carotene, fatty acid C18:2 and vitamin B6 were inversely associated with unchanged/increased GC dose (OR 0.29–0.30 (95% CI 0.10 to 0.90)), whereas vitamin B12 and calcium were associated with unchanged/increased GC dose (OR 3.20–5.36 (95% CI 1.08 to 17.52)). Omega-3 fatty acids and the ratio between omega-6 and omega-3 fatty acids were not significantly associated with unchanged/increased GC dose.
The association between dietary nutrient intake and GC dose change (decreased vs unchanged/increased) between –1 year to inclusion, inclusion to +1 year and −1 year to +1 year
Total energy intake was associated with GC doses >5.0 mg/day and >7.5 mg/day, explaining an association between 35 nutrients and higher GC dose levels (OR 2.98–23.82 (95% CI 1.01 to 203.88)).
Discussion
This study focused on the association between diet and GC treatment in patients with SLE. The results were based on data from a Swedish SLE cohort and showed that some dietary nutrients were associated or inversely associated with unfavourable outcomes of GC.
Diet and GC treatment
Several previous studies have shown that low vitamin D levels in serum are associated with disease activity in SLE.12 ,17–20 Vitamin D supplementation has also been suggested to improve disease activity and fatigue scores in SLE.21 ,22 Based on these and other studies, it has been suggested that vitamin D is protective against lupus activity. However, in this study, dietary vitamin D was positively associated with GC treatment, not suggesting a protective effect, but this association was not significant after adjusting for supplement use of calcium/vitamin D supplementation. Patients included in this study might have been aware of the existing evidence of the beneficial effect of vitamin D, explaining an increased vitamin D intake in patients with GC treatment/higher disease activity. Nevertheless, these results were based on dietary intake and not supplement intake of vitamin D. The results on the association between dietary vitamin D and GC were affected by calcium/vitamin D supplementation. However, if an adequate vitamin D intake is obtained (either through diet or supplementation), it is not necessarily followed by adequate vitamin D levels in blood, since GC reduces absorption of vitamin D.23
Alcohol was inversely associated with GC treatment, reflecting older findings on the link between moderate alcohol intake and reduced risk of rheumatic diseases,24 ,25 and even specifically of SLE.13–15
Diet and unchanged/increased GC dose
Fatty acid C18:2 (linoleic acid) was inversely associated with unchanged/increased GC dose (unfavourable outcome). Fatty acid C18:2 belongs to the omega-6 family and is known to have proinflammatory properties. However, the omega-3 fatty acids (anti-inflammatory) should be considered when looking at the omega-6 intake since the ratio between these two must be well balanced. The omega-6 to omega-3 fatty acid ratio did not show any association with unchanged/increased GC dose. Though, a review has gathered evidence on health benefits of conjugated linoleic acids26 that may be linked with the inverse association between dietary linoleic acid and unchanged/increased GC dose.
An inverse association was found between beta-carotene and unchanged/increased GC dose. Beta-carotene is an antioxidant and patients with SLE have been found to have lower dietary beta-carotene intake compared with controls.27 Increased antioxidant intake could be beneficial due to the damage of free oxygen radicals that play a role in SLE.28 ,29 Beta-carotene may be linked to protective effect against unchanged/increased GC dose.
Vitamin B6 was inversely associated with unchanged/increased GC dose. Higher intake of vitamin B6 and dietary fibre may prevent the occurrence of active disease in patients with SLE.6 Pyridoxal 5′-phosphate (PLP) is the active coenzyme form of vitamin B6 and its concentration in plasma is the most common measure of vitamin B6 status.30 ,31 Decreased plasma PLP levels have shown to be associated with chronic or acute disease and increased plasma PLP levels with lower C reactive protein.32–34 Increased dietary vitamin B6 intake may play a role in lupus activity.
Diet and GC dose
Energy intake was associated with higher GC dose levels, explaining the association between almost all the nutrients and higher GC dose levels. Contradictory results exist on the effects of GC therapy on energy intake, appetite and body weight in humans.35 GC has shown to be associated with increased appetite and/or body weight.36 Increased appetite during or after GC treatment has been self-reported as one of the major adverse events in several studies.37–39 This study confirms the existing evidence of GC's influence on increasing appetite.
Limitations
Patients included in this study had various disease and treatment durations at inclusion. GC treatment may not have fully reflected lupus activity during the 2-year period. Some micronutrients, when consumed together, interfere with each other in the physiological environment; however, interactions between nutrients as well as nutrient bioavailability were not taken into account. Dietary data from FFQ were based on estimated dietary consumption. Recall bias and under-reporting and over-reporting may have occurred when completing the FFQ. Also, dietary patterns were assumed to be the same throughout the 2-year period. Clinical manifestations, treatment history and side effects of GC throughout the disease course from diagnosis were not considered in this study.
Conclusions
These data did not support the hypothesis that dietary vitamin D protects against lupus activity. Beta-carotene (antioxidant), fatty acid C18:2 (omega-6) and vitamin B6 may protect against unfavourable outcomes (need for increases in GC dose). The inverse association between alcohol intake and GC treatment/lupus activity may provide a partial explanation for the link between moderate alcohol intake and improved cardiovascular health in rheumatic diseases. The association between dietary intake and higher GC dose levels indicated GC's influence on increasing appetite.
References
Footnotes
Contributors CL collected data on GC treatment from medical records and performed all statistical analyses. JF provided data on dietary intake from SLEVIC master file. RvV played a major role in study design and drafting the manuscript together with CL. JF and IH were instrumental in establishing the SLEVIC registry and contributed to study design and interpretation. All authors approved the final manuscript.
Competing interests None declared.
Patient consent Obtained.
Ethics approval This study was approved by regional ethical review board at Karolinska Institutet, Stockholm, Sweden.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.