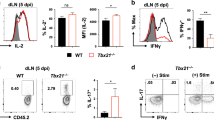
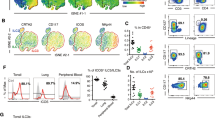
Abstract
We examined the requirement for and cooperation between CD28 and inducible costimulator (ICOS) in effective T helper (TH) cell responses in vivo. We found that both CD28 and ICOS were critical in determining the outcome of an immune response; cytolytic T lymphocyte–associated antigen 4–immunoglobulin (CTLA-4–Ig), ICOS-Ig and/or a neutralizing ICOS monoclonal antibody attenuated T cell expansion, TH2 cytokine production and eosinophilic inflammation. CD28-dependent signaling was essential during priming, whereas ICOS–B7RP-1 regulated TH effector responses, and the up-regulation of chemokine receptors that determine T cell migration. Our data suggests a scenario whereby both molecules regulate the outcome of the immune response but play separate key roles: CD28 primes T cells and ICOS regulates effector responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Schwartz, R. H. A cell culture model for T lymphocyte clonal anergy. Science 4961, 1349–1356 (1990).
Coyle, A. J. & Gutierrez-Ramos, J. C. The expanding B7 superfamily: Increasing complexity in costimulatory signals regulating T cell function. Nature Immunol . 2, 1–7 (2001).
Harding, F. A., McArthur, J. G., Gross, J. A., Raulet, D. H. & Allison, J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 6370, 607–609 (1992).
Jenkins, M. K., Taylor, P. S., Norton, S. D. & Urdahl, K. B. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J. Immunol. 8, 2461–2466 (1991).
Lucas, P. J., Negishi, I., Nakayama, K., Fields, L. E. & Loh, D. Y. Naive CD28-deficient T cells can initiate but not sustain an in vitro antigen-specific immune response. J. Immunol. 154, 5757–5768 (1997).
Garside, P. et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science 281, 96–99 (1998).
Walunas, T. L. et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1, 405–413 (1994).
Tivol, E. A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 (1995).
Hutloff, A. et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 6716, 263–267 (1999).
Coyle, A. J. J. et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity 1, 95–105 (2000).
Yoshinaga, S. K. et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature 402, 827–832 (1999).
Swallow, M. M., Wallin, J. J. & Sha, W. C. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNF-α. Immunity 11, 423–432 (1999).
Rulifson, I. C., Sperling, A. I., Fields, P. E., Fitch, F. W. & Bluestone, J. A. CD28 costimulation promotes the production of Th2 cytokines. J. Immunol. 15, 658–665 (1997)
Dong, C. et al. ICOS costimulatory receptor is essential for T-cell activation and function. Nature 409, 97–101 (2001).
McAdam, A. J., Greenwald, R. J., Levin, M. A., Freeman, G. J. & Sharpe, A. H. ICOS is critical for CD40 mediated antibody class switching. Nature 409, 102–105 (2001).
Tafuri, A. et al. Essential role of ICOS in effective helper T cell responses. Nature 409, 105–109 (2001).
Baggiolini, M. Chemokines and leukocyte traffic. Nature 392, 565–568 (1998).
Sallusto, F., Lenig, D., Mackay, C. R. & Lanzavecchia, A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 187, 875–883 (1998).
Bonecchi, R. et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187, 129–134 (1998).
Sallusto, F., Mackay, C. R. & Lanzavecchia, A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science 277, 2005–2007 (1998).
Shahinian, A. et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261 609–612 (1993).
Tada, Y. et al. CD28-deficient mice are highly resistant to collagen-induced arthritis. J. Immunol. 162, 203–208 (1999).
Girvin, A. M. et al. A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J. Immunol. 164, 136–143 (2000).
Mathur, M. et al. CD28 interactions with either CD80 or CD86 are sufficient to induce allergic airway inflammation in mice. Am. J. Respir. Cell Mol. Biol. 21, 498–509 (1999).
Linsley, P.S. et al. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science 257, 792–795 (1992).
Borriello, F. et al. B7–1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 6, 303–313 (1997).
Tang, A., Judge, T. A., Nickoloff, B. J. & Turka, L. A. Suppression of murine allergic contact dermatitis by CTLA-4-Ig. Tolerance induction of Th2 responses requires additional blockade of CD40-ligand. J. Immunol. 1, 117–125 (1996).
Gause, W. C. et al. polygyrus: B7-independence of the secondary type 2 response. Exp. Parasitol. 84, 264–273 (1996).
McAdam, A. J. et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4(+) T cells. J. Immunol. 165, 5035–5040 (2000).
Corry, D. B., Reiner, S. L., Linsley, P. S. & Locksley, R. M. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J. Immunol. 9, 4142–4148 (1994).
Lenschow, D. J. et al. CD28/B7 regulation of TH1 and TH2 subsets in the development of autoimmune diabetes. Immunity 3, 285–293 (1996).
Gonzalo, J. A., Delaney, T., Corcoran, J., Gutierrez-Ramos, J. C. & Coyle, A. J. Complimentary and unique signals delivered by ICOS and CD28 regulate T cell effector function. J. Immunol. 166, 1–5 (2001).
Rottman, J. B. et al. The costimulatory molecule ICOS plays an important role in central nervous system demyelination. Nature Immunol. 2, 605–611 (2001).
Ozkaynak, E. et al. Importance of ICOS–B7RP-1 costimulation in acute and chronic allograft rejection. Nature Immunol. 2, 591–596 (2001).
Gonzalo, J. A. et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J. Exp. Med. 188, 157–167 (1998).
Gonzalo, J. A. et al. The expression of eotaxin parallels eosinophil accumulation and is not restricted to Th2 type response in the lung. Immunity 4, 1–14 (1996).
Coyle, A. J., Lloyd, C. M. & Gutierrez-Ramos, J. C. Biotherapeutic targets for the treatment of allergic airway disease. Am. J. Respir. Crit. Care. Med. 162, 179–184 (2000).
Tsuyuki, S., Tsuyuki, J., Einsle, K., Kopf, M. & Coyle, A. J. Costimulation through B7–2 (CD86) is required for the induction of a lung mucosal T helper cell 2 (TH2) immune response and altered airway responsiveness. J. Exp. Med. 185, 1671–1679 (1997).
Keane-Myers, A., Gause, W. C., Linsley, P. S., Chen, S. J. & Wills-Karp, M. B7-CD28/CTLA-4 costimulatory pathways are required for the development of T helper cell 2-mediated allergic airway responses to inhaled antigens. J. Immunol . 158, 2042–2049 (1997).
Schweitzer, A. N. & Sharpe, A. H. Studies using antigen-presenting cells lacking expression of both B7–1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J. Immunol. 6, 2762–2771 (1998).
London, C. A., Lodge, M. P. & Abbas, A. K. Functional responses and costimulator dependence of memory CD4+ T cells. J. Immunol. 164, 265–272 (2000).
Schaerli, P. et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192, 1553–1562 (2000).
Breitfeld, D. et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192, 1545–1552 (2000).
Mackay, C. R. Follicular homing T helper (Th) cells and the TH1/TH2 paradigm. J. Exp. Med. 192, 31–34 (2000).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Kopf, M. et al. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J. Exp. Med. 192, 111–117 (2000).
Walker, L.S. et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J. Exp. Med. 190, 1115–1122 (1999).
Bretscher, P. A. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc. Natl Acad. Sci. USA 96, 185–190 (1999).
Lloyd, C. M. et al. CC chemokine receptor (CCR)3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J. Exp. Med. 191, 265–274 (2000).
Gonzalo, J. A. et al. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J. Immunol. 163, 403–411 (1999).
Chensue, S. W. et al. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in cc chemokine receptor 8 knockout mice. J. Exp. Med. 193, 573–584 (2001).
Murphy, K.M. et al. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 21, 1720–1723 (1990).
Acknowledgements
We thank K. McDonald for purification of ICOS-Ig, C. Groves for advice on FACS analysis and S. Manning for his contributions to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonzalo, J., Tian, J., Delaney, T. et al. ICOS is critical for T helper cell–mediated lung mucosal inflammatory responses. Nat Immunol 2, 597–604 (2001). https://doi.org/10.1038/89739
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/89739
This article is cited by
-
Acazicolcept (ALPN-101), a dual ICOS/CD28 antagonist, demonstrates efficacy in systemic sclerosis preclinical mouse models
Arthritis Research & Therapy (2022)
-
Targeting co-stimulatory molecules in autoimmune disease
Nature Reviews Drug Discovery (2020)
-
Modulating local airway immune responses to treat allergic asthma: lessons from experimental models and human studies
Seminars in Immunopathology (2020)
-
Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses
Cellular & Molecular Immunology (2019)
-
Cell-surface molecule-mediated cell–cell interactions in the regulation of ILC2-driven allergic inflammation
Cellular and Molecular Life Sciences (2019)