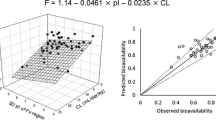
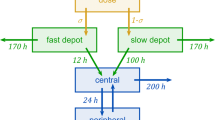
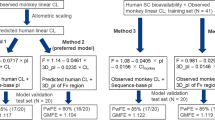
Abstract
The subcutaneous (SC) route is of growing interest for the administration of biotherapeutics. Key products on the biotherapeutic market such as insulins, but also several immunoglobulins or Fc-fusion proteins, are administered SC. Despite the importance of the SC route, the available knowledge about the processes involved in the SC absorption of biotherapeutics is limited. This review summarizes available information on the physiology of the SC tissue and on the pharmacokinetic processes after SC administration including “first pass catabolism” at the administration site as well as transport in the extracellular matrix of the SC tissue, followed by absorption into the blood circulation or the lymphatic system. Both monoclonal antibodies and other biotherapeutics are discussed. Determinants of absorption after SC administration are summarized including compound properties such as charge or molecular weight. Scale-up of animal data to humans is discussed, including the current shortcomings of empirical scaling approaches and the lack of suitable mechanistic approaches.






Similar content being viewed by others
REFERENCES
Tang L, Persky AM, Hochhaus G, Meibohm B. Pharmacokinetic aspects of biotechnology products. J Pharm Sci. 2004;93(9):2184–204.
McDonald TA, Zepeda ML, Tomlinson MJ, Bee WH, Ivens IA. Subcutaneous administration of biotherapeutics: current experience in animal models. Curr Opin Mol Ther. 2010;12(4):461–70.
Hale G, Rebello P, Brettman LR, Fegan C, Kennedy B, Kimby E, et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood. 2004;104(4):948–55.
Porter CJ, Charman SA. Lymphatic transport of proteins after subcutaneous administration. J Pharm Sci. 2000;89(3):297–310.
Buttel IC, Chamberlain P, Chowers Y, Ehmann F, Greinacher A, Jefferis R, et al. Taking immunogenicity assessment of therapeutic proteins to the next level. Biologicals. 2011;39(2):100–9.
Supersaxo A, Hein WR, Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res. 1990;7(2):167–9.
Lewis JH. The route and rate of absorption of subcutaneously injected serum in relation to the occurrence of sudden death after injection of antitoxic horse serum. JAMA. 1921;76:1342–5.
Field ME, Drinker CK. The permeability of the capillaries of the dog to protein. Am J Physiol. 1931;97:40–51.
Weinstein JN, Steller MA, Covell DG, Holton OD, Keenan AM, Sieber SM, et al. Monoclonal antitumor antibodies in the lymphatics. Cancer Treat Rep. 1984;68(1):257–64.
Gibney MA, Arce CH, Byron KJ, Hirsch LJ. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin. 2010;26(6):1519–30.
Mirrashed F, Sharp JC, Krause V, Morgan J, Tomanek B. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10(3):161–8.
Lancerotto L, Stecco C, Macchi V, Porzionato A, Stecco A, De Caro R. Layers of the abdominal wall: anatomical investigation of subcutaneous tissue and superficial fascia. Surg Radiol Anat. 2011;33(10):835–42.
Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73(1):1–78.
Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31(12):446–51.
Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007;4(4):427–40.
Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50(1–2):3–20.
Kretsos K, Kasting GB. Dermal capillary clearance: physiology and modeling. Skin Pharmacol Physiol. 2005;18(2):55–74.
Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng. 2007;9:229–56.
Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller GA, Lim JE, et al. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release. 2006;114(2):230–41.
Reed RK, Lepsoe S, Wiig H. Interstitial exclusion of albumin in rat dermis and subcutis in over- and dehydration. Am J Physiol. 1989;257(6 Pt 2):H1819–27.
Parker JC, Gilchrist S, Cartledge JT. Plasma-lymph exchange and interstitial distribution volumes of charged macromolecules in the lung. J Appl Physiol. 1985;59(4):1128–36.
Mrsny RJ. Metabolic processes at injection sites affecting pharmacokinetics, pharmacodynamics, and metabolism of protein and peptide therapeutics. In: Mrsny RJ, Daugherty A, editors. Proteins and peptides—pharmacokinetics, harmacodynamic, and metabolic outcomes. New York: Informa healthcare; 2009.
Braverman IM, Keh-Yen A. Ultrastructure of the human dermal microcirculation. III. The vessels in the mid- and lower dermis and subcutaneous fat. J Investig Dermatol. 1981;77(3):297–304.
Ryan TJ, Mortimer PS, Jones RL. Lymphatics of the skin. Neglected but important. Int J Dermatol. 1986;25(7):411–9.
Schacht V, Luedemann W, Abels C, Berens von Rautenfeld D. Anatomy of the subcutaneous lymph vascular network of the human leg in relation to the great saphenous vein. Anat Rec (Hoboken). 2009;292(1):87–93.
Leak LV. Electron microscopic observations on lymphatic capillaries and the structural components of the connective tissue-lymph interface. Microvasc Res. 1970;2(4):361–91.
Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. J Investig Dermatol Symp Proc. 2000;5(1):14–9.
Charman SA, McLennan DN, Edwards GA, Porter CJ. Lymphatic absorption is a significant contributor to the subcutaneous bioavailability of insulin in a sheep model. Pharm Res. 2001;18(11):1620–6.
Reddy ST, Berk DA, Jain RK, Swartz MA. A sensitive in vivo model for quantifying interstitial convective transport of injected macromolecules and nanoparticles. J Appl Physiol. 2006;101(4):1162–9.
Beshyah SA, Anyaoku V, Niththyananthan R, Sharp P, Johnston DG. The effect of subcutaneous injection site on absorption of human growth hormone: abdomen versus thigh. Clin Endocrinol (Oxf). 1991;35(5):409–12.
Patel TV, Robinson K, Singh AK. Is it time to reconsider subcutaneous administration of epoetin? Nephrol News Issues. 2007;21(11):57. 9, 63–4 passim.
ter Braak EW, Woodworth JR, Bianchi R, Cerimele B, Erkelens DW, Thijssen JH, et al. Injection site effects on the pharmacokinetics and glucodynamics of insulin lispro and regular insulin. Diabetes Care. 1996;19(12):1437–40.
Jensen JD, Jensen LW, Madsen JK. The pharmacokinetics of recombinant human erythropoietin after subcutaneous injection at different sites. Eur J Clin Pharmacol. 1994;46(4):333–7.
Yoshikawa H, Satoh Y, Naruse N, Takada K, Muranishi S. Comparison of disappearance from blood and lymphatic delivery of human fibroblast interferon in rat by different administration routes. J Pharmacobiodyn. 1985;8(3):206–10.
Binder C. Absorption of injected insulin. A clinical–pharmacological study. Acta Pharmacol Toxicol (Copenh). 1969;27 Suppl 2:1–84.
Xu Z, Wang Q, Zhuang Y, Frederick B, Yan H, Bouman-Thio E, et al. Subcutaneous bioavailability of golimumab at 3 different injection sites in healthy subjects. J Clin Pharmacol. 2010;50(3):276–84.
McLennan DN, Porter CJ, Edwards GA, Martin SW, Heatherington AC, Charman SA. Lymphatic absorption is the primary contributor to the systemic availability of epoetin alfa following subcutaneous administration to sheep. J Pharmacol Exp Ther. 2005;313(1):345–51.
Charman SA, Segrave AM, Edwards GA, Porter CJ. Systemic availability and lymphatic transport of human growth hormone administered by subcutaneous injection. J Pharm Sci. 2000;89(2):168–77.
McLennan DN, Porter CJ, Edwards GA, Brumm M, Martin SW, Charman SA. Pharmacokinetic model to describe the lymphatic absorption of r-metHu-leptin after subcutaneous injection to sheep. Pharm Res. 2003;20(8):1156–62.
Kagan L, Gershkovich P, Mendelman A, Amsili S, Ezov N, Hoffman A. The role of the lymphatic system in subcutaneous absorption of macromolecules in the rat model. Eur J Pharm Biopharm. 2007;67(3):759–65.
Kota J, Machavaram KK, McLennan DN, Edwards GA, Porter CJ, Charman SA. Lymphatic absorption of subcutaneously administered proteins: influence of different injection sites on the absorption of darbepoetin alfa using a sheep model. Drug Metab Dispos. 2007;35(12):2211–7.
Kagan L, Turner MR, Balu-Iyer SV, Mager DE. Subcutaneous absorption of monoclonal antibodies: role of dose, site of injection, and injection volume on rituximab pharmacokinetics in rats. Pharm Res. 2012;29(2):490–9.
Oussoren C, Storm G. Liposomes to target the lymphatics by subcutaneous administration. Adv Drug Deliv Rev. 2001;50(1–2):143–56.
Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–58.
McLennan DN, Porter CJ, Edwards GA, Heatherington AC, Martin SW, Charman SA. The absorption of darbepoetin alfa occurs predominantly via the lymphatics following subcutaneous administration to sheep. Pharm Res. 2006;23(9):2060–6.
Chen SA, Sawchuk RJ, Brundage RC, Horvath C, Mendenhall HV, Gunther RA, et al. Plasma and lymph pharmacokinetics of recombinant human interleukin-2 and polyethylene glycol-modified interleukin-2 in pigs. J Pharmacol Exp Ther. 2000;293(1):248–59.
Porter CJ, Edwards GA, Charman SA. Lymphatic transport of proteins after s.c. injection: implications of animal model selection. Adv Drug Deliv Rev. 2001;50(1–2):157–71.
Supersaxo A, Hein W, Gallati H, Steffen H. Recombinant human interferon alpha-2a: delivery to lymphoid tissue by selected modes of application. Pharm Res. 1988;5(8):472–6.
Bocci V, Muscettola M, Grasso G, Magyar Z, Naldini A, Szabo G. The lymphatic route. 1) Albumin and hyaluronidase modify the normal distribution of interferon in lymph and plasma. Experientia. 1986;42(4):432–3.
Kojima K, Takahashi T, Nakanishi Y. Lymphatic transport of recombinant human tumor necrosis factor in rats. J Pharmacobiodyn. 1988;11(10):700–6.
Wu F, Bhansali SG, Tamhane M, Kumar R, Vathy LA, Ding H, et al. Noninvasive real-time fluorescence imaging of the lymphatic uptake of BSA-IRDye 680 conjugate administered subcutaneously in mice. J Pharm Sci. 2012;101:1744–54.
Hawley AE, Davis SS, Illum L. Targeting of colloids to lymph nodes: influence of lymphatic physiology and colloidal characteristics. Adv Drug Deliv Rev. 1995;17:129–48.
Xie DD, Hale V. Factors affecting the lymphatic absorption of macromolecules following extravascular administration. Pharm Res. 1996;13:S396.
Patel HM, Boodle KM, Vaughan-Jones R. Assessment of the potential uses of liposomes for lymphoscintigraphy and lymphatic drug delivery. Failure of 99 m-technetium marker to represent intact liposomes in lymph nodes. Biochim Biophys Acta. 1984;801(1):76–86.
Kaur CD, Nahar M, Jain NK. Lymphatic targeting of zidovudine using surface-engineered liposomes. J Drug Target. 2008;16(10):798–805.
Kaledin VI, Matienko NA, Nikolin VP, Gruntenko YV, Budker VG, Vakhrusheva TE. Subcutaneously injected radiolabeled liposomes: transport to the lymph nodes in mice. J Nat Cancer Inst. 1982;69(1):67–71.
Mangat S, Patel HM. Lymph node localization of non-specific antibody-coated liposomes. Life Sci. 1985;36(20):1917–25.
Trubetskoy VS, Whiteman KR, Torchilin VP, Wolf GL. Massage-induced release of subcutaneously injected liposome-encapsulated drugs to the blood. J Control Release. 1998;50(1–3):13–9.
Olszewski W, Engeset A, Jaeger PM, Sokolowski J, Theodorsen L. Flow and composition of leg lymph in normal men during venous stasis, muscular activity and local hyperthermia. Acta Physiol Scand. 1977;99(2):149–55.
Astrup A, Bulow J, Madsen J. Skin temperature and subcutaneous adipose blood flow in man. Scand J Clin Lab Invest. 1980;40(2):135–8.
O'Morchoe CC, Jones 3rd WR, Jarosz HM, O'Morchoe PJ, Fox LM. Temperature dependence of protein transport across lymphatic endothelium in vitro. J Cell Biol. 1984;98(2):629–40.
Bhansali SG, Balu-Iyer SV, Morris ME. Influence of route of administration and liposomal encapsulation on blood and lymph node exposure to the protein VEGF-C156S. J Pharm Sci. 2012;101(2):852–9.
Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493–507.
Oh CK, Faggioni R, Jin F, Roskos LK, Wang B, Birrell C, et al. An open-label, single-dose bioavailability study of the pharmacokinetics of CAT-354 after subcutaneous and intravenous administration in healthy males. Br J Clin Pharmacol. 2010;69(6):645–55.
Law B, Tung CH. Proteolysis: a biological process adapted in drug delivery, therapy, and imaging. Bioconjug Chem. 2009;20(9):1683–95.
Berger M, Halban PA, Girardier L, Seydoux J, Offord RE, Renold AE. Absorption kinetics of subcutaneously injected insulin. Evidence for degradation at the injection site. Diabetologia. 1979;17(2):97–9.
Watanabe RM, Volund A, Bergman RN. Intravenous insulin infusion to simulate subcutaneous absorption. Bioavailability and metabolic sequelae. Diabetes Care. 1991;14(11):1021–30.
Wang W, Prueksaritanont T. Prediction of human clearance of therapeutic proteins: simple allometric scaling method revisited. Biopharm Drug Dispos. 2010;31(4):253–63.
Deng R, Loyet KM, Lien S, Iyer S, DeForge LE, Theil FP, et al. Pharmacokinetics of humanized monoclonal anti-tumor necrosis factor-{alpha} antibody and its neonatal Fc receptor variants in mice and cynomolgus monkeys. Drug Metab Dispos. 2010;38(4):600–5.
Mortensen DL, Walicke PA, Wang X, Kwon P, Kuebler P, Gottlieb AB, et al. Pharmacokinetics and pharmacodynamics of multiple weekly subcutaneous efalizumab doses in patients with plaque psoriasis. J Clin Pharmacol. 2005;45(3):286–98.
Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs. 2011;3(1):61–6.
Kawamata S, Ozawa J, Hashimoto M, Kurose T, Shinohara H. Structure of the rat subcutaneous connective tissue in relation to its sliding mechanism. Arch Histol Cytol. 2003;66(3):273–9.
Yoshioka E, Kato K, Shindo H, Mitsuoka C, Kitajima SI, Ogata H, et al. Pharmacokinetic study of darbepoetin alfa: absorption, distribution, and excretion after a single intravenous and subcutaneous administration to rats. Xenobiotica. 2007;37(1):74–90.
Martin SM, O'Donnell RT, Kukis DL, Abbey CK, McKnight H, Sutcliffe JL, et al. Imaging and pharmacokinetics of (64)Cu-DOTA-HB22.7 administered by intravenous, intraperitoneal, or subcutaneous injection to mice bearing non-Hodgkin's lymphoma xenografts. Mol Imaging Biol. 2009;11(2):79–87.
Rose EH, Vistnes LM, Ksander GA. The panniculus carnosus in the domestic pig. Plast Reconstr Surg. 1977;59(1):94–7.
Wells MY, Voute H, Bellingard V, Fisch C, Boulifard V, George C, et al. Histomorphology and vascular lesions in dorsal rat skin used as injection sites for a subcutaneous toxicity study. Toxicol Pathol. 2010;38(2):258–66.
Harvey AJ, Kaestner SA, Sutter DE, Harvey NG, Mikszta JA, Pettis RJ. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharm Res. 2011;28(1):107–16.
Woo S, Jusko WJ. Interspecies comparisons of pharmacokinetics and pharmacodynamics of recombinant human erythropoietin. Drug Metab Dispos. 2007;35(9):1672–8.
Zheng Y, Tesar DB, Benincosa L, Birnbock H, Boswell CA, Bumbaca D, et al. Minipig as a potential translatable model for monoclonal antibody pharmacokinetics after intravenous and subcutaneous administration. MAbs. 2012;4(2):243–55.
Hoffman A, Ziv E. Pharmacokinetic considerations of new insulin formulations and routes of administration. Clin Pharmacokinet. 1997;33(4):285–301.
Grahnen A, Kastrup K, Heinrich U, Gourmelen M, Preece MA, Vaccarello MA, et al. Pharmacokinetics of recombinant human insulin-like growth factor I given subcutaneously to healthy volunteers and to patients with growth hormone receptor deficiency. Acta Paediatr Suppl. 1993;82 Suppl 391:9–13. discussion 4.
van Gils FC, Westerman Y, van den Bos C, Burger H, van Leen RW, Wagemaker G. Pharmacokinetic basis for optimal hemopoietic effectiveness of homologous IL-3 administered to rhesus monkeys. Leukemia. 1993;7(10):1602–7.
Biesma B, Pokorny R, Kovarik JM, Duffy FA, Willemse PH, Mulder NH, et al. Pharmacokinetics of recombinant human interleukin 3 administered subcutaneously and by continuous intravenous infusion in patients after chemotherapy for ovarian cancer. Cancer Res. 1993;53(24):5915–9.
Konrad MW, Hemstreet G, Hersh EM, Mansell PW, Mertelsmann R, Kolitz JE, et al. Pharmacokinetics of recombinant interleukin 2 in humans. Cancer Res. 1990;50(7):2009–17.
Tanaka H, Tanaka Y, Shinagawa K, Yamagishi Y, Ohtaki K, Asano K. Three types of recombinant human granulocyte colony-stimulating factor have equivalent biological activities in monkeys. Cytokine. 1997;9(5):360–9.
Radwanski E, Chakraborty A, Van Wart S, Huhn RD, Cutler DL, Affrime MB, et al. Pharmacokinetics and leukocyte responses of recombinant human interleukin-10. Pharm Res. 1998;15(12):1895–901.
Aoyama K, Uchida T, Takanuki F, Usui T, Watanabe T, Higuchi S, et al. Pharmacokinetics of recombinant human interleukin-11 (rhIL-11) in healthy male subjects. Br J Clin Pharmacol. 1997;43(6):571–8.
Wills RJ, Dennis S, Spiegel HE, Gibson DM, Nadler PI. Interferon kinetics and adverse reactions after intravenous, intramuscular, and subcutaneous injection. Clin Pharmacol Ther. 1984;35(5):722–7.
Segrave AM, Mager DE, Charman SA, Edwards GA, Porter CJ. Pharmacokinetics of recombinant human leukemia inhibitory factor in sheep. J Pharmacol Exp Ther. 2004;309(3):1085–92.
Laursen T, Grandjean B, Jorgensen JO, Christiansen JS. Bioavailability and bioactivity of three different doses of nasal growth hormone (GH) administered to GH-deficient patients: comparison with intravenous and subcutaneous administration. Eur J Endocrinol. 1996;135(3):309–15.
Bocci V, Muscettola M, Naldini A. The lymphatic route—III. Pharmacokinetics of human natural interferon-beta injected with albumin as a retarder in rabbits. Gen Pharmacol. 1986;17(4):445–8.
Salmonson T, Danielson BG, Wikstrom B. The pharmacokinetics of recombinant human erythropoietin after intravenous and subcutaneous administration to healthy subjects. Br J Clin Pharmacol. 1990;29(6):709–13.
Ramakrishnan R, Cheung WK, Wacholtz MC, Minton N, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after single and multiple doses in healthy volunteers. J Clin Pharmacol. 2004;44(9):991–1002.
Woo S, Krzyzanski W, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after intravenous and subcutaneous administration in rats. J Pharmacol Exp Ther. 2006;319(3):1297–306.
Ramakrishnan R, Cheung WK, Farrell F, Joffee L, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after intravenous and subcutaneous dose administration in cynomolgus monkeys. J Pharmacol Exp Ther. 2003;306(1):324–31.
Karlsson MO, Wade JR, Loumaye E, Munafo A. The population pharmacokinetics of recombinant—and urinary—human follicle stimulating hormone in women. Br J Clin Pharmacol. 1998;45(1):13–20.
Macdougall IC, Gray SJ, Elston O, Breen C, Jenkins B, Browne J, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol. 1999;10(11):2392–5.
Liles D, Landen CN, Monroe DM, Lindley CM, Read MS, Roberts HR, et al. Extravascular administration of factor IX: potential for replacement therapy of canine and human hemophilia B. Thromb Haemost. 1997;77(5):944–8.
McCarthy K, Stewart P, Sigman J, Read M, Keith Jr JC, Brinkhous KM, et al. Pharmacokinetics of recombinant factor IX after intravenous and subcutaneous administration in dogs and cynomolgus monkeys. Thromb Haemost. 2002;87(5):824–30.
Joshi A, Bauer R, Kuebler P, White M, Leddy C, Compton P, et al. An overview of the pharmacokinetics and pharmacodynamics of efalizumab: a monoclonal antibody approved for use in psoriasis. J Clin Pharmacol. 2006;46(1):10–20.
ACKNOWLEDGMENTS
This study was supported in part by the Center for Protein Therapeutics, University at Buffalo, State University of New York (for MEM). The authors thank Elke Atzpodien, F. Hoffmann-La Roche, Basel, for providing the section of rat skin.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Craig Svensson, Joseph Balthasar, and Frank-Peter Theil
Rights and permissions
About this article
Cite this article
Richter, W.F., Bhansali, S.G. & Morris, M.E. Mechanistic Determinants of Biotherapeutics Absorption Following SC Administration. AAPS J 14, 559–570 (2012). https://doi.org/10.1208/s12248-012-9367-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9367-0