Article Text
Abstract
Objectives Patients with systemic lupus erythematosus (SLE) are at higher risk of haematological malignancies (HMs) than the general population. Most reports have focused on HM diagnosed after SLE, and have excluded concurrent and preceding diagnoses. Information on response to therapy is also limited.
Methods We identified 13 296 cases of HM and 10 539 potential patients with SLE at our centre; 45 patients were confirmed to have HM and SLE. Our retrospective case series was based on these 45 patients.
Results Of the 45 patients, 64% were diagnosed with HM ≥1 year after diagnosis with SLE, and 36% with HM before or concurrent with SLE. Of the 29 patients with HM after SLE, 13 had diffuse large B cell lymphoma (DLBCL), 6 indolent lymphoma, 4 leukaemia, 3 Hodgkin's disease, and 1 each Burkitt's lymphoma, T cell lymphoma and multiple myeloma. Eleven patients with DLBCL were treated with cyclophosphamide, hydroxydaunorubicin, oncovin and prednisone (CHOP) or rituximab-CHOP; hydroxydaunorubicin, oncovin and prednisone; only four achieved durable remission. Of the 16 patients diagnosed with HM before or concurrent with SLE, 9 were diagnosed with HM more than 2 years before SLE and tended to be in remission prior to SLE diagnosis. Seven patients were diagnosed with HM and SLE concurrently; in terms of their HM, six achieved remission or stable disease.
Conclusions In summary, DLBCL was the most common type of lymphoma in patients diagnosed with HM after SLE; these patients presented with advanced-stage disease and had poor outcomes. In contrast, patients diagnosed with HM before or concurrent with SLE had early stage disease and typically achieved remission.
- Systemic Lupus Erythematosus
- Hematologic neoplasms
- Diffuse large B-cell lymphoma
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
-
Patients with diffuse large B-cell lymphoma typically presented with advanced-stage disease despite being under observation for their SLE.
-
One-third of patients were diagnosed with a haematological malignancy before or concurrent with SLE, a group that has not been previously characterised.
-
For concurrent patients, SLE features seemed to bring the haematological malignancy to clinical attention early, possibly resulting in better outcomes.
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that preferentially affects women from early adulthood through mid-adulthood.1 SLE is characterised by autoantibody formation against nuclear antigens with resultant inflammation in organs such as the kidney, skin and joints. SLE is notorious for its markedly heterogeneous clinical phenotype, with a multifactorial aetiology that depends on genetic, epigenetic and environmental influences.2 While mortality attributable to disease manifestations and their treatment have improved, patients with SLE continue to die from cardiovascular disease, infections and cancer.3 ,4 In general, patients with autoimmune disease are at increased risk for haematological malignancies (HMs).5–12 This is certainly true for patients with SLE who appear to be at higher risk for cancer in general,13 ,14 and especially non-Hodgkin's lymphoma (NHL),15 a cause of increased mortality in patients with SLE.16 It has been suggested that patients with pre-existing autoimmune disease will have a lower 5-year survival if diagnosed with lymphoma.17
Relatively little is known about HM risk factors and response to treatment in patients with SLE, although such questions have begun to be addressed in small series.18–20 The roles of immunosuppressive medications and disease activity also continue to be debated,21–23 without a clear consensus. Here, we were interested in better understanding the clinical details and response to treatment of patients with SLE and HM. The outcome of patients with both diagnoses has only been considered to a limited extent in the literature, with relatively little known about histopathology other than diffuse large B cell lymphoma (DLBCL).24 Also, studies have typically focused on patients diagnosed with HM after SLE, with concurrent and preceding diagnoses of HM excluded. Although there have been many hypothesised links between SLE and lymphoma, including a common genetic predisposition, chronic stimulation of the immune system and disproportional immune responses,13 ,14 ,23 the aetiology of the increased risk of HM in patients with SLE remains unclear. We therefore sought to characterise all patients at a single tertiary centre who carried diagnoses of SLE and HM, independent of the sequence and relative timing of the two diagnoses.
Methods
Patient identification
At the University of Michigan, patients with a diagnosis of malignancy are tracked by the Tumor Registry. To identify patients with HM, we searched the registry for all patients with an International Classification of Diseases (ICD)-O-3 code of 9599–9999; this strategy included patients with lymphoma, plasma cell tumours, leukaemia, myeloproliferative disorders and myelodysplastic syndromes (MDSs). We also searched University of Michigan billing and laboratory databases to identify patients who might have a diagnosis of SLE; these data sources include inpatient and outpatient records. Specifically, the billing database was searched for patients with an ICD-9 code of 710.0, and the laboratory database was searched for patients with a positive double-stranded DNA (dsDNA) antibody test (by a radioimmunoassay performed in the University of Michigan Department of Pathology clinical laboratory). In our experience with lupus surveillance, we have found that case finding using ICD codes coupled with laboratory results successfully identifies the vast majority of patients with SLE. We cross-linked results from the two searches to identify patients who may have been diagnosed with HM and SLE.
Clinical data and chart review
The medical records of patients who might carry a diagnosis of HM and SLE were reviewed in detail, first extracting data regarding the SLE diagnosis. Data pertinent to the American College of Rheumatology (ACR) classification of SLE were abstracted.25 Additionally, we collected data on the timing of SLE diagnosis, medication exposures and coexisting diagnoses such as Sjögren's syndrome and rheumatoid arthritis. Only patients who met four or more ACR criteria for SLE were considered further.25
In terms of the HM diagnosis, all histopathology was reviewed by a University of Michigan haematopathologist. International Prognostic Index scores (which predict a worse prognosis with age >60 years, Ann Arbor stage III or IV disease, elevated lactate dehydrogenase (LDH), poor performance status or more than one extranodal site) were calculated whenever possible. Ann Arbor staging roughly defines stage I as involving a single lymph node region; stage II as involving multiple regions on the same side of the diaphragm; stage III as involving lymph node regions on both sides of the diaphragm; and stage IV as demonstrating diffuse or disseminated involvement of at least one extralymphatic organ.26 ,27 In the International Prognostic Index calculation, hospitalisation at the time of diagnosis was used as a surrogate marker of poor performance status. Response to treatment was determined from the treating physician’s clinical documentation. The University of Michigan institutional review board reviewed and approved this study.
Statistical analysis
Summary statistics were computed for continuous measures as mean±SD or median (IQR) if not normally distributed. For categorical variables, frequency and proportion (%) were determined. After assessing for normality, variables were log-transformed if needed. Two-sample t tests were used to compare the equality of means between continuous variables, and χ2 tests to compare categorical variables. A histogram plot was used to display the distribution and sequence of time between SLE and HM diagnoses.
Results
Identification and characterisation of patients with SLE and HM
We identified 13 296 cases of HM in the University of Michigan Tumor Registry and 10 539 patients within the University of Michigan system with either a billing code for SLE or a positive dsDNA antibody test, and therefore a possible diagnosis of SLE. Data were available from 1980 onwards for the Tumor Registry and 1988 onwards for the billing and laboratory databases. After linking results from the two searches, 130 patients were identified in common. Of these 130 patients, 45 met four or more ACR criteria for SLE.25 Of the remaining 85 cases, the majority (71/85) did not have SLE and were simply miscoded; some of the miscoded patients had other rheumatological diagnoses such as Sjögren's syndrome, rheumatoid arthritis and dermatomyositis. For a minority of patients (14/85), SLE was considered, but the diagnosis was not definitive, with either a better alternative diagnosis present (such as Sjögren's syndrome) or an inability on chart review to identify four ACR criteria (usually positive lab testing, but an absence of clinical features). Overall, this level of false positives is consistent with what we have found in lupus surveillance where up to 60% of screened patients are false positives on detailed chart review.1 Of the 45 validated SLE cases, two were diagnosed with HM in the 1980s, 11 in the 1990s and the remainder 2000 onwards. This increase in patient numbers with each successive decade coincides with growth in patient volume at the University of Michigan Health System during this time (greater than twofold increase in patient encounters), and steady annual increases in the patient population with SLE at the institution.
The characteristics of the 45 patients with validated SLE are listed in table 1. As expected, the majority of patients were female (84%). Most patients also had a positive antinuclear antibody (ANA) using a standard human epithelial type 2 (HEp-2) substrate and the University of Michigan laboratory cut-off of 1:80. Approximately half of the patients had positive dsDNA antibodies and antiphospholipid antibodies (at least one of anticardiolipin, anti-β2GPI (beta-2 glycoprotein I) or lupus anticoagulant), respectively. In terms of SLE manifestations, arthritis (87%) and haematological abnormalities (84%) were most common. Most patients were treated with prednisone (76%) and antimalarial agents (73%), while approximately half were treated with immunosuppression. Seven patients had coexisting Sjögren's syndrome, while no patient had coexisting rheumatoid arthritis; the only patients with rheumatoid factor (RF) positivity had coexisting Sjögren's syndrome which was the presumed source of the positive RF. The seven patients with Sjögren's syndrome included three cases of mucosa-associated lymphoid tissue (MALT) lymphoma, and one case each of aplastic anaemia, DLBCL, follicular lymphoma and small lymphocytic lymphoma. Two patients had received a kidney transplant for end-stage renal disease secondary to SLE, and their HM is probably best classified as post-transplant lymphoproliferative disorder (PTLD); these two patients were also considered in our study of PTLD at the University of Michigan.28
Demographic and clinical characteristics of patients with systemic lupus erythematosus (SLE) and a haematological malignancy (HM)*
Relative timing of SLE and HM diagnoses
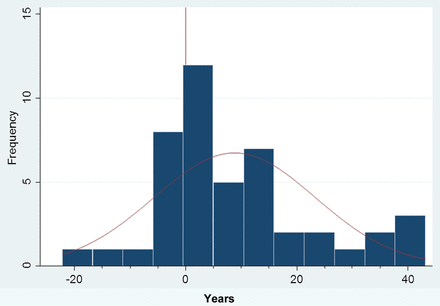
Of the 45 patients with HM and validated SLE, 29 were diagnosed with HM one or more years after diagnosis with SLE; the remaining 16 patients were diagnosed with HM concurrently or before SLE (figure 1). These groups are considered separately in table 1. Of note, patients diagnosed with HM after SLE were more likely to be diagnosed with SLE earlier in life and HM later in life, to have lupus-specific autoantibodies and to have haematological manifestations of their SLE.
Time between systemic lupus erythematosus (SLE) and haematological malignancy diagnoses. For each patient, the time between diagnoses of SLE and haematological malignancy was determined in years. A positive value indicates that SLE was diagnosed before the haematological malignancy. A negative value indicates the haematological malignancy was diagnosed first.
Patients diagnosed with HM after SLE
Tumour histopathology for the HM-after-SLE and the HM-before/concurrent groups are described in table 2. As expected, DLBCL was the most common diagnosis in the 29 patients with HM after SLE (45%). The characteristics of these 13 DLBCLs are presented in more detail in table 3. Four patients with DLBCL were treated with cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone (CHOP) and seven with rituximab-CHOP; taking these 11 together, six (55%) died (all within 14 months), one (9%) had active disease at last follow-up (11 months) and four (36%) achieved durable remission (range 28–102 months). Both of the aforementioned patients who had a renal transplant had DLBCL histopathology; one had disease limited to the kidney which responded to reduction in immunosuppression (lymphoma in remission for 76 months at last follow-up, although with loss of the allograft), while the other had stage IV disease and died within 1 month of lymphoma diagnosis.
Haematological malignancy (HM) histopathology
Characteristics of diffuse large B cell lymphoma (DLBCL) diagnosed after systemic lupus erythematosus (SLE)
Sixteen of the 29 patients with HM after SLE had a diagnosis other than DLBCL (table 2). All three cases of Hodgkin's lymphoma had Ann Arbor stage II or III disease. Two responded well to treatment with standard adriamycin, bleomycin, vinblastine and dacarbazine chemotherapy and achieved durable remission (40 months and 51 months, respectively, at last follow-up). The other patient was too ill for chemotherapy and died 2 months after diagnosis. Of the six cases of indolent lymphoma, two were chronic lymphocytic leukaemia/small lymphocytic lymphoma, two were MALT lymphoma, one was Waldenström's macroglobulinemia, and one was follicular lymphoma. Three patients were treated with chemotherapy, while the others received either local therapy or were simply observed. Outcomes were good with mean survival of 39 months at last follow-up (range 18–112 months); one patient died, unrelated to their stable HM or lupus.
The one patient with Burkitt's lymphoma had an aggressive disease course refractory to multiple types of chemotherapy; the patient died 4 months after diagnosis. The patient with stage III angioimmunoblastic T cell lymphoma achieved remission with CHOP chemotherapy, but then had relapse of disease 67 months later. The patient with multiple myeloma was treated with multiple chemotherapeutic agents including bortezomib, lenalidomide, prednisone, doxorubicin HCl liposome and cyclophosphamide, as well as autologous peripheral blood stem cell transplant; the myeloma had a progressive and relapsing disease course, but the patient was nevertheless still alive at 58 months of follow-up. Four patients had bone marrow dyscrasias, including two cases of acute myelogenous leukaemia (AML), one case of MDS, and one case of aplastic anaemia; three of these patients died of either their disease or complications of graft-versus-host disease. The patient with aplastic anaemia achieved durable remission (41 months) with allogeneic stem cell transplant.
Patients diagnosed with HM before SLE
Of the 16 patients diagnosed with HM before or concurrent with SLE, nine (56%) were diagnosed with HM more than 2 years before SLE (mean 7 years; range 2–22); these patients tended to have early-stage HM and were in remission prior to SLE diagnosis. No particular HM histology predominated with one case of Hodgkin's lymphoma (stage II), one low-grade plasmacytoid lymphoma (stage II), one MALT lymphoma (stage II), two DLBCL (both stage IE), one peripheral T cell lymphoma (stage IE), one cutaneous T cell lymphoma, one T cell large granular lymphocytic (LGL) leukaemia, and one MDS. The patients with Hodgkin's lymphoma, plasmacytoid lymphoma, and MALT lymphoma were treated with external-beam radiation therapy as the sole means of therapy; all achieved durable remission (mean 208 months; range 115–304). Two additional patients within this group (one with DLBCL and one with T cell lymphoma) also received external-beam radiation as part of their remission-inducing regimen; these were also the only two patients to receive cytotoxic chemotherapy prior to the diagnosis of SLE. Two patients, one with T cell LGL leukaemia and one with MDS, were treated with observation alone, for 3 years and 5 years, respectively, before the diagnosis of SLE was made. The patient with T cell LGL leukaemia had a particularly complicated past medical history that also included a diagnosis of cirrhosis secondary to hepatitis C, treated with two liver transplants.
For these nine patients in whom SLE was diagnosed more than 2 years after HM, ANA was positive in nine (100%), dsDNA antibodies in three (33%) and antiphospholipid antibodies in three (33%); clinical manifestations were notable for haematological abnormalities in six (67%), arthritis in five (56%) and nephritis in three (33%). Despite a history of malignancy, five patients (56%) were treated with immunosuppressive treatment targeting their SLE, including two patients with cyclophosphamide (both had nephritis). One of the patients treated with cyclophosphamide did have relapse of their DLBCL 9 months after beginning immunosuppressive treatment (remission had been present for 54 months) and ultimately sought palliative treatment. There was no evidence of HM relapse or progression in any of the other patients, despite diagnosis with SLE.
Patients diagnosed with HM and SLE concurrently
Seven patients were diagnosed with HM and SLE concurrently, meaning the two diagnoses occurred within 1 year of each other (mean difference 4 months; range 0–10). Again, no particular type of HM predominated with one case of Hodgkin's lymphoma (stage III), one MALT lymphoma (stage IV), one cutaneous T cell lymphoma, one multiple myeloma, two AML and one Janus-associated kinase 2-positive myeloproliferative disorder. Five patients (71%) were treated with chemotherapy. One of the patients with AML died while undergoing treatment for leukaemia progression 23 months after diagnosis. The other six patients (86%) had an average survival of 48 months (range 34–80 months), all with remission or stable disease at last follow-up. As in table 4, all seven patients had serology consistent with SLE including all seven with a positive ANA. The most common clinical manifestations were arthritis (100%) and haematological abnormalities (71%); no patient had nephritis. In terms of SLE treatment, six patients were treated with hydroxychloroquine, while only one required traditional immunosuppression (methotrexate) targeting the SLE.
Characteristics of patients diagnosed with a haematological malignancy (HM) and systemic lupus erythematosus (SLE) concurrently*
Discussion
The link between SLE and cancer dates back to the 1970s, with the publication of case series of non-HMs29 and lymphoma30 in patients with SLE. In the 1990s, a number of SLE cohorts were described, with the majority of studies suggesting only a borderline risk of cancer in general, but a statistically significant risk of NHL (relative risk ranging from 4 to 44).31–34 The increased risk of NHL was not, however, observed in all cohorts.35 ,36
In 2005, more definitive statistics were provided by two studies: a meta-analysis of lymphoma risk in patients with SLE,5 and a large international cohort of 9547 patients with SLE.37 The meta-analysis considered six cohort studies and found a standardised incidence ratio (SIR) of 7.4 (95% CI 3.3 to 17.0) for NHL in SLE; this was in comparison with a SIR of 18.8 (95% CI 9.5 to 37.3) for Sjögren's syndrome and 3.9 (95% CI 2.5 to 5.9) for rheumatoid arthritis, determined by similar methodology.5 The international cohort demonstrated a SIR of 1.15 (95% CI 1.05 to 1.27) for all cancers combined, but 3.64 (95% CI 2.63 to 4.93) for NHL;37 a subsequent analysis of the NHL cases from the international cohort suggested that 52% were best classified as DLBCL by histopathology.38 This international cohort was recently updated (now including 16 409 patients with SLE) with a SIR of 4.39 for NHL (95% CI 3.46 to 5.49).15
In keeping with previous studies, DLBCL was the most common type of histopathology in our population, especially in the subgroup diagnosed with HM after SLE (45% of these patients). These patients presented with advanced-stage and extranodal disease, and had relatively poor outcomes despite aggressive treatment. It does not appear that the increased clinical attention these patients received for their SLE resulted in earlier diagnosis or better outcomes. It should also be pointed out that the two patients with PTLD met criteria for the DLBCL group and its subsequent analysis; one of these patients had limited disease that responded to a reduction in immunosuppression, and the other was started on CHOP chemotherapy and had active disease at last follow-up.
A strength of our work is that we identified a number of cases of HM diagnosed either before or concurrently with SLE; such cases have frequently been excluded from previous studies. As expected, the patients with HM more than 2 years before SLE had less aggressive types of HM that permitted survival to the time of their SLE diagnosis. The history of HM did not clearly impact on treatment of their SLE, and patients were treated with aggressive treatment (such as cyclophosphamide for lupus nephritis) when indicated. Only one of the nine patients had relapse of their HM.
We were also interested in the group of patients diagnosed with HM and SLE concurrently. These patients all met four or more ACR criteria for SLE, and one wonders whether this presentation might best be considered a paraneoplastic syndrome; for example, ANAs have been described in conjunction with HM.39 ,40 Having said that, most patients were treated with either hydroxychloroquine or methotrexate, suggesting that the physicians taking care of the patients at the time were not satisfied that their lupus-like symptoms would simply respond to treatment of the HM. From an HM perspective, the outcomes of these concurrent patients were good, and we speculate that their SLE symptoms (such as arthritis) might have brought their HM to clinical light earlier. Of course, it is also possible that SLE symptoms received increased attention as a result of the HM diagnosis. A larger population of patients will be needed to comment further on these possibilities.
This study has limitations, including its retrospective nature. In some patients, follow-up after HM diagnosis was as long as 304 months, although the mean was 61 months, and the median 40 months. When commenting on the stability of HM remission, longer follow-up could impact our findings. Also, given the limited number of patients available for study, definitive statistical analysis of risk factors is not possible; however, our hope is that this descriptive report and some of the novel subgroups described will foster additional population-based and prospective research. It should also be pointed out that the percentage of patients exhibiting haematological criteria here (84%) is somewhat higher than has been found in the general lupus population (>65%),1 and it is possible that some of these abnormalities may be more attributable to the HM than the SLE.
Unlike rheumatoid arthritis, where medications such as methotrexate and possibly tumour necrosis factor inhibitors are felt to play an important role in predisposition to HM,41 ,42 the aetiology underlying the association between HM and SLE remains much less clear. In SLE, the roles of immunosuppressive medications and disease activity remain to be fully characterised.21–23 Shared genetic risk and other environmental factors should also be further explored.
In summary, we have identified a population of patients with HM and SLE at a single tertiary care centre. A third of these patients were diagnosed with HM before or concurrently with SLE, a group that has typically not been included in previous studies. Our data suggest that SLE features may bring comorbid HM to clinical attention early, possibly resulting in better outcomes. In contrast, DLBCL usually presented after SLE, and these patients presented with advanced-stage disease despite being under observation for SLE.
References
Footnotes
-
Contributors All authors made substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data, to drafting the work or revising it critically for important intellectual content, gave final approval of the version submitted and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
-
Funding JSK was funded in part by Training Grant T32 AR7080-33. JSK was also supported by career development awards from the Rheumatology Research Foundation and the Burroughs Wellcome Fund. ECS was supported by K01 ES019909 from NIH, and an Arthritis Foundation Health Professional New Investigator Award.
-
Competing interests None.
-
Ethics approval The University of Michigan institutional review board reviewed and approved this study (HUM00006319).
-
Provenance and peer review Not commissioned; externally peer reviewed.
-
Data sharing statement No additional data are available.