Article Text
Abstract
Objective To describe the pharmacodynamic monitoring of (immuno)proteasome inhibition following treatment with bortezomib in a therapy-refractory systemic lupus erythematosus (SLE) patient with life-threatening myocarditis and lupus nephritis.
Patient and methods Inhibition of catalytic activities of the proteasome subunits β5 (constitutive proteasome), β5i and β1i (immunoproteasome) were measured in peripheral blood mononuclear cells using subunit-specific fluorogenic peptide substrates in a patient who received three cycles of bortezomib (1.3 mg/m2 subcutaneously, days 1, 4, 8 and 11; every three weeks) along with plasma exchange during the first two cycles.
Results Proteasome β5, β5i and β1i subunit activities were readily inhibited 1 h after bortezomib administration. Twenty-four hours post-bortezomib administration, β5 and β5i activities were largely restored, whereas inhibition of β1i activity was sustained. Clinically, after three cycles, cardiac function had improved, with concurrent improvement of haemodynamic stability during haemodialysis. Anti-ds-DNA dropped from >400 to 12 IU/mL along with normalisation of complement C3 and C4. Bortezomib therapy was well tolerated, and patient now has a sustained remission for >16 months.
Conclusions This case illustrates the potential benefit of pharmacodynamic monitoring of (immune)proteasome subunit-specific activity after bortezomib dosing in patients with therapy refractory SLE. This tool may hold potential to guide personalised/precision dosing aiming to achieve maximal efficacy and minimal toxicity.
- Systemic Lupus Erythematosus
- Lupus Nephritis
- B cells
- Pharmacokinetics
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with heterogeneous presentation and involvement of multiple organ systems, resulting in high morbidity and a threefold higher mortality rate than the general population. Myocarditis is an uncommon manifestation and occurs particularly in conjunction with pericarditis. Active nephropathy is observed in nearly 30% of patients with SLE and is associated with a further increase in mortality risk.1 To date, beyond conventional immunosuppressive agents, various biologicals are used for therapy: rituximab and belimumab (targeting B cells), abatacept (inhibition of T cell activation) and eculizumab (interfering in the complement cascade), demonstrating variable efficacies.2 In spite of these therapies, a subgroup of patients with SLE are refractory to treatment and experience increasing morbidity due to ongoing disease activity and/or drug toxicity.
Proteasome inhibitors have been identified as a novel experimental treatment modality based on their mechanisms of action (depletion of long-lived plasma cells and inhibitory effects on critical signalling pathways) and have encouraging effects in animal models with lupus-like disease.3–5 The proteasome inhibitor bortezomib has been approved for the treatment of multiple myeloma and mantle cell lymphoma6 and has also been successfully applied in a small group of refractory patients with SLE7 and two patients with SLE with concomitant multiple myeloma.8 These observations warrant further clinical evaluation of bortezomib treatment in patients with refractory SLE, ideally in combination with pharmacodynamic end point assessments. Pharmacokinetic testing in multiple myeloma showed that subcutaneous administration of bortezomib confers similar area under the curve concentrations as intravenous administration, but with much lower peak levels and subsequently reduction of side effects (eg, polyneuropathy). Pharmacodynamic monitoring in these trials was performed by measuring the inhibition of the activity of the constitutive proteasome subunit β5 by bortezomib.9 Autoimmune diseases like SLE, however, are characterised by upregulation of immunoproteasome subunits.10 ,11 New assays are now available to measure the specific catalytic activity of the subunits of the immunoproteasome, and these assays could be of value to optimise dosing of bortezomib in patients with SLE. Here, as a feasibility study, we measured the specific activity of the immunoproteasome subunits β5i and β1i, as well as the constitutive proteasome subunit β5, in blood cells during bortezomib treatment.
Materials and methods
Catalytic activity of (immuno)proteasome subunits
When feasible, blood samples were drawn prior to bortezomib therapy and 1 and 24 h after bortezomib administration during consecutive cycles of bortezomib. Peripheral blood mononuclear cells (PBMCs) were harvested by Ficoll density gradient centrifugation and stored at −80°C until analysis. Catalytic activity of constitutive proteasome subunit β5 and the immunoproteasome subunits β5i and β1i was analysed in cell extracts of PBMCs using specific fluorogenic peptide substrates; Ac-WLA-AMC, Ac-ANW-AMC and Ac-PAL-AMC, respectively, essentially as described previously.12
Patient history
A 43-year-old male patient from South American origin was diagnosed with SLE in 2009, based on pericarditis, arthritis, lymphadenopathy and positive autoimmune serology (antinuclear, anti-dsDNA, anti-Sm and anti-RNP antibodies). He had a history of persistent disease (arthritis, myopathy and lymphadenopathy) under successive treatments with hydroxychloroquine, azathioprine, rituximab and mycophenolate mofetil (MMF) in combination with prednisolone and courses of methylprednisolone (MPNS). Despite this treatment, he was diagnosed with proliferative lupus nephritis (ISN/RPS class IV-G) in August 2012 for which he was treated with cyclophosphamide according to the ‘Eurolupus’ regimen and subsequently with MMF. With this treatment, his kidney function recovered and urinalysis normalised.
In April 2013, he developed heart failure. A myocardial biopsy showed evidence of myocarditis with abundant infiltration of macrophages next to lymphocytes (figure 1). He was treated with MPNS and intravenous immunoglobulin. This resulted in a temporary clinical response, but in November 2013 he was admitted to the intensive care unit with respiratory failure caused by heart failure and acute kidney failure, despite maintenance therapy with corticoids and MMF. The acute kidney failure was induced by a flare of lupus nephritis (proteinuria increased to 1.6 g/day), probably concomitant with acute tubular necrosis due to heart failure. Treatment included non-invasive ventilation, renal replacement therapy and MPNS. At this stage, experimental therapy with bortezomib (1.3 mg/m2, subcutaneous, days 1, 4, 8 and 11; every three weeks) was started because of the otherwise expected fatal outcome, in combination with plasma exchange during the first two cycles. Bortezomib therapy was well tolerated (except for transient thrombocytopenia during the first cycle) and effective. After three cycles, cardiac function had improved, along with normalisation of anti-ds-DNA levels (>400 to 12 IU/mL) and complement C3 and C4 (figure 2). Maintenance therapy consisted of MMF 1000 mg twice daily and low-dose prednisolone. The patient now has a sustained remission for almost 2 years.
Immunohistochemical staining of myocardial tissue before start of bortezomib treatment. (A) Lymphocytes (CD45) and (B) macrophages (CD68). Magnification ×200.
Time course for laboratory parameters of a patient with systemic lupus erythematosus prior and during bortezomib therapy (arrow). (A) Anti-ds-DNA (normal range: <15 IU/mL), (B) erythrocyte sedimentation rate (ESR; normal: <10 mm/h), (C) complement C3 (normal range: 0.9–1.8 g/L) and (D) complement C4 (normal range: 0.15–0.4 g/L).
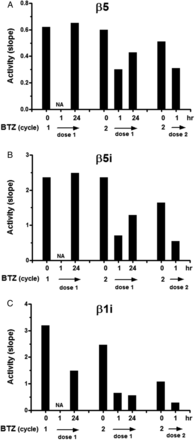
From blood samples drawn during the first two cycles, pharmacodynamic monitoring of inhibition of catalytic activity of individual proteasome subunits known to be targeted by bortezomib, that is, constitutive β5 and the immunoproteasome subunits β5i and β1i, was assessed (figure 3). In PBMCs, 1 h after bortezomib administration, β5-activity was suppressed (mean 45% compared with untreated controls), but this activity was largely recovered 24 h later. Likewise, immunoproteasome β5i catalytic activity was potently inhibited 1 h after drug administration (mean 73% compared with untreated controls). After 24 h, residual β5i inhibition was 25% compared with untreated control. Finally, β1i activity was also potently inhibited 1 h after bortezomib administration (mean 74% compared with untreated controls), but remarkably, this inhibition was largely sustained (mean 65% compared with untreated controls) over 24 h.
Bortezomib (BTZ)-induced inhibition of (immuno)proteasome activity in peripheral blood cells of a patient with systemic lupus erythematosus . Catalytic activity of β5, β5i and β1i is depicted at three time points during bortezomib treatment either prior to bortezomib dosing and 1 and 24 h post-bortezomib administration. NA, sample not available.
Discussion
In this study, we describe for the first time the dynamics of immunoproteasome inhibition in this patient with SLE by assessment of bortezomib-induced inhibition in PBMCs of the catalytic activities associated with the β5i and β1i immunoproteasome subunits, next to the β5 constitutive subunit. Consistent with bortezomib being a reversible proteasome inhibitor,3 inhibition of β5 and β5i shortly after bortezomib administration was largely relieved after 24 h. Interestingly, dynamics of β1i catalytic activity showed a different profile. First, inhibition by bortezomib was sustained for >24 h. Second, basal β1i activity appears to decrease during the course of bortezomib. The latter could reflect the loss of immune-competent cells with aberrant β1i activity during treatment. In this regard, Ghannam et al10 showed that active inflammation in myositis was associated with upregulation of β1i expression. Similarly, Morawietz et al11 showed that expression of β1i is significantly increased in inflammatory infiltrates of salivary glands in patients with Sjogren’s syndrome. As bortezomib targeting may involve various immune cells (B cells, plasma cells, T cells, macrophages and dendritic cells),3 ,4 ,13 it is conceivable that inhibition of β1i activity therein contributes to bortezomib's therapeutic effect, for example, by induction of apoptosis, suppression of pro-inflammatory cytokine release and/or altered generation of antigenic peptides with a consequently lower autoimmune response.
Together with the first two cycles of bortezomib, our patient received plasma exchanges that could have contributed to his recovery. Although plasma exchange is still recommended in life-threatening SLE disease activity, several studies did not show any benefits of plasma exchanges in patients with active lupus nephritis.14 Moreover, the sustained clinical response makes a plasma exchange-induced improvement less likely.
Given the fact that bortezomib therapy showed efficacy by inducing remission of disease activity in several individual cases and large case series of patients with therapy-refractory SLE,7 ,8 ,15 further exploration of proteasome inhibitor-based therapies is warranted.
Although bortezomib therapy over three cycles was well tolerated by our patient (except for transient thrombocytopenia), awareness of potential toxic side effects should be considered in case of repeated treatments.6 Specific adverse events may include peripheral neuropathy, thrombocytopenia, diarrhoea and infectious complications, among which the latter were reported by Alexander and colleagues in a series of 12 patients with SLE treated with bortezomib7 according to schedules applied for multiple myeloma treatment.6 ,15
Assessment of ‘molecular therapeutic efficacy’ by measuring (immuno)proteasome subunit inhibition, particularly of the β1i subunit, would be helpful to design optimal dosing strategies in future clinical studies with bortezomib to achieve maximal efficacy and minimal toxicity for patients with refractory SLE.
Acknowledgments
The authors thank Johan van Meerloo for technical assistance.
References
Footnotes
Contributors Design of study: JWvdH, SZ and GJ. Clinical data acquisition: KAdG, MTS, HMWN, SZ, AEV and JWvdH. Conducted experiments: DN, JC and GJ. Contributed to reagents and analytic tools: DN and JLB. Data analysis: KAdG, DN, JC, HWMN, GJ and JWvdH. Writing of the manuscript: KAdG, SZ, AEV, GJ and JWvdH.
Competing interests SZ reports grant from Janssen, Celgene and Takeda, outside the submitted work. JC reports speakers fees from Takeda, outside the submitted work. JLB is employee of Takeda Oncology.
Provenance and peer review Not commissioned; externally peer reviewed.