Article Text
Abstract
Objective Fatigue has been reported as the most disturbing symptom in a majority of patients with SLE. Depression is common and often severe. Together these symptoms cause significant morbidity and affect patients with otherwise relatively mild disease. Tryptophan and its metabolites in the kynurenine pathway are known to be important in several psychiatric conditions, for example, depression, which are often also associated with fatigue. We therefore investigated the kynurenine pathway in patients with SLE and controls.
Methods In a cross-sectional design plasma samples from 132 well-characterised patients with SLE and 30 age-matched and gender-matched population-based controls were analysed by liquid chromatography tandem mass spectrometry to measure the levels of tryptophan and its metabolites kynurenine and quinolinic acid. Fatigue was measured with Fatigue Severity Scale and depression with Hospital Anxiety and Depression Scale. SLE disease activity was assessed with Systemic Lupus Erythematosus Disease Activity Index (SLEDAI).
Results The kynurenine/tryptophan ratio, as a measure of indoleamine 2,3-dioxygenase (IDO) activity, was increased in patients with SLE. Patients with active disease (SLEDAI ≥6) showed lower tryptophan levels compared with controls (54 µM, SD=19 vs 62 µM, SD=14, p=0.03), although patients with SLE overall did not differ compared with controls. Patients with SLE had higher levels of tryptophan metabolites kynurenine (966 nM, SD=530) and quinolinic acid (546 nM, SD=480) compared with controls (kynurenine: 712 nM, SD=230, p=0.0001; quinolinic acid: 380 nM, SD=150, p=0.001). Kynurenine, quinolinic acid and the kynurenine/tryptophan ratio correlated weakly with severe fatigue (rs =0.34, rs =0.28 and rs =0.24, respectively) but not with depression.
Conclusions Metabolites in the kynurenine pathway are altered in patients with SLE compared with controls. Interestingly, fatigue correlated weakly with measures of enhanced tryptophan metabolism, while depression did not. Drugs targeting enzymes in the kynurenine pathway, for example, IDO inhibitors or niacin (B12) supplementation, which suppresses IDO activity, merit further investigation as treatments in SLE.
- systemic lupus erythematosus
- autoimmune diseases
- autoimmunity
- disease activity
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Depression and cognitive dysfunction are the most common psychiatric manifestations in SLE. Depression is present in 11%–39% of the patients,1 and many patients with SLE also suffer from anxiety.2 3 Fatigue has been reported to be present in up to 90% of women with SLE4 and when given open questions, a majority reported fatigue as the most troublesome SLE-related symptom.2 5 Fatigue is sometimes also known to be associated with symptoms of depression, but the underlying mechanisms are unknown.6 7 A large fraction of patients with SLE experience fatigue in the absence of psychiatric disorders. Possible connections between fatigue and SLE disease activity have been studied but with contradictory results.8
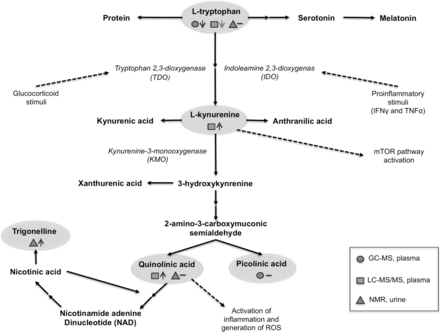
The kynurenine pathway is known to be upregulated as part of an activated immune response,9 and recently the tryptophan metabolism via the kynurenine pathway has been highlighted as a mechanism of central fatigue.10 11 The kynurenine pathway has an established role in depression where low plasma levels of tryptophan and elevated levels of metabolites, that is, increased kynurenine/tryptophan ratio, have been detected in the circulation and in the cerebrospinal fluid (CSF).12 In the kynurenine pathway (figure 1), two enzymes, tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3-dioxygenases (IDO), are responsible for the first step where tryptophan is converted to kynurenine. Kynurenine can further be converted to picolinic acid or quinolinic acid, and quinolinic acid to nicotinic acid.13 IDO is mainly present in immune cells and known to be induced by interferon gamma (IFN-γ), interleukin (IL-2), IL-1β, prostaglandin E2, oxidative stress and LPS.14 TDO is mainly expressed in the liver and regulated by the levels of tryptophan and by glucocorticoids.15 16 Higher kynurenine/tryptophan ratios, as a measure of IDO activity, have been reported in patients with SLE.17–19
The kynurenic pathway. The encircled metabolites were measured and symbols indicate method and biological sample, that is, LC-MS/MS detection in plasma, NMR detection in urine and GC-MS detection in plasma. Our results comparing SLE and controls are represented by a hyphen if no change in metabolite level and an upward pointing arrow if the metabolite level was increased in SLE. Our hypothesis for a possible role of the kynurenic pathway in the pathogenesis of SLE is illustrated by dashed arrows. LC-MS/MS, liquid chromatography tandem mass spectrometry; GC-MS, gas chromatography mass spectrometry; NMR, nuclear magnetic resonance (NMR); ROS, reactive oxygen species; TNF-α, tumour necrosis factor alpha; mTOR, mammalian target of rapamycin .
In this study, we investigated levels of tryptophan and its metabolites in the kynurenine pathway in patients with SLE and in controls. Among patients with SLE, associations with fatigue, depression and disease activity were evaluated.
Material and methods
Study design and cohort
EDTA plasma and urine samples from a cross-sectional SLE cohort at Karolinska University Hospital, Stockholm, Sweden, were used in this study. All patients with SLE were adults (>18 years old) and fulfilled four of the American College of Rheumatology (ACR) 1982 revised classification criteria for SLE.20 Samples were selected to be representative for the Karolinska SLE cohort as previously described,21 and in addition, samples from a pilot study were included.22 In total, 132 samples and 32 age-matched and gender-matched population controls were selected for analysis of the kynurenine pathway.
Clinical assessments
To assess anxiety and depression, the participants answered the questionnaire Hospital Anxiety and Depression Scale (HADS).23 24 HADS consists of 14 items; each item is weighted from 0 to 3, giving a possible sum ranging from 0 to 21 for the anxiety scale and depression scale, respectively.23 The participants also filled in the Fatigue Severity Scale (FSS).25 26 FSS is a nine-item scale scored from one to seven; the mean value of the answered questions results in the final score (range 1–7). The cut-off for severe fatigue have been suggested to be ≥4,27 and mean score for healthy adults varies between 2.3 (SD=0.7) and 3.0 (SD=1.08).25 28 In this study, we denote a score of 1–3 as ‘no/limited fatigue’ and a score of ≥4 as ‘severe fatigue’. Disease activity at sampling was defined by Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)29 and Systemic Lupus Activity Measures (SLAM).30 Active disease was defined as SLEDAI ≥6 or SLAM ≥7.31 The Systemic Lupus International Collaboration Clinics (SLICC)/ACR damage index was used to assess organ damage.30
Analysis of tryptophan, kynurenine and quinolinic acid by liquid chromatography tandem mass spectrometry
An aliquot of 25 µL EDTA plasma was mixed with an internal standard solution containing 13C3 15N1-quinolinic acid, D4-kynurenine, D5-tryptophan and D5-kynurenic acid and filtered before injection into a HPLC system. Detection was performed using a triple quadrupole mass spectrometer targeting tryptophan, kynurenine, quinolinic acid and the corresponding internal standards as previously described.32 Quantification was performed using internal standard and Masslynx 4.1 software (Waters Corporation). Two controls failed in the analysis, and thus this study is based on a subcohort of 132 patients with SLE and 30 controls.
Analysis of tryptophan and picolinic acid by gas chromatography mass spectrometry (GC-MS)
Plasma EDTA samples from patients with SLE (n=114) and controls (n=23) from our cohort were analysed by GC-MS for metabolomics profiling.21 Samples were randomly selected to batches, and three out of four batches were successfully analysed.21 Data regarding tryptophan and picolinic acid from these 87 patients and 18 controls were used in this work.
Analysis of tryptophan, quinolinic acid and trigonelline by nuclear magnetic resonance (NMR) spectroscopy
Urine samples from 58 patients with SLE and 12 controls were analysed for metabolite content by NMR in another project. The samples to be analysed by NMR in that project were selected based on the results from the GC-MS analysis described above. Samples in batches 1 and 3 from the GC-MS analysis were analysed by NMR since these two batches showed the largest difference in metabolic profile when comparing SLE and control. For each sample, 250 µL urine was mixed with 250 µL phosphate - buffered saline (PBS) (pH 7.4) containing 3 mM NaN3 and 5.5 mM trimethylsilyl propanoic acid (for chemical shift and line-shape calibration) and then transferred to 5 mm NMR tubes. The analysis was performed on a Bruker 600MHz Avance III HD spectrometer equipped with a BBO cryoprobe with Z-gradients and a SampleJet sample changer (Bruker Biospin, Rheinstetten, Germany). The samples were kept at +6°C prior to analysis, and the temperature during the experiment was +27°C. A 1D NOESY presaturation experiment (pulse sequence noesygppr1d) with 64 scans and a relaxation delay (d1) of 4 s was performed on each sample. Metabolite identification and quantification was performed using Chenomx NMR suite 8.1 (www.chenomx.com), and for each sample, the quantified metabolite concentrations were normalised to concentration of creatinine. Two samples (one control and one SLE) were excluded from further analysis due to significantly different NMR spectra as compared with rest of the samples.
Analysis of cytokines by mesoscale
Cytokines were analysed in EDTA plasma samples from the SLE cohort using the MSD V-PLEX Human Cytokine 30-plex kit (K15054D; Mesoscale Discovery, Gaithersburg, Maryland, USA)33 and cytokines, which previously have been suggested to be increased in patients with SLE,34–37 that is, tumour necrosis factor alpha (TNF-α), IFN-γ-induced protein 10 (IP-10), interleukin (IL)-16, macrophage inflammatory proteins 1 beta (MIP-1β), and IL-6 were studied in this work.
Statistics
Unpaired t-test with Welch correction or non-parametric Mann-Whitney U test were performed for normally and non-normally distributed data, respectively. Differences were considered significant at p<0.05. The Spearman’s rank-order correlation coefficient (rs ) was used to measure the strength of the linear association between two variables. No adjustment for multiple testing has been applied in this work, but we consider comparisons with p<0.001 with higher confidence.
Results
The kynurenine pathway is altered in patients with SLE
Characteristics of the investigated patients with SLE and controls are reported in table 1. Comparing patients with controls, we obtained significantly higher levels of kynurenine, quinolinic acid, as well as the kynurenine/tryptophan ratio in plasma from patients with SLE, while levels of tryptophan did not differ (table 2). However, when the active SLE group was compared with controls, the tryptophan levels were significantly higher in controls compared with active disease (SLEDAI ≥6 vs control: p=0.03; SLAM ≥7 vs control: p=0.04). Both patients with active or inactive disease, according to SLEDAI, had higher levels of kynurenine, quinolinic acid and higher kynurenine/tryptophan ratio compared with controls (table 2). Higher levels of kynurenine and kynurenine/tryptophan ratio were also seen when active disease was defined by SLAM. However, none of the metabolites correlated (rs <0.2) with SLAM or SLEDAI, neither when the whole SLE group nor when active disease only was considered.
Characteristics of patients with SLE and controls
Metabolites in kynurenine pathway (LC-MS/MS data) in SLE, controls and SLE subgroups
In the SLE cohort (n=132), 80 patients were treated with prednisolone. Higher levels of tryptophan were detected in patients with prednisolone treatment compared with patients without prednisolone (60 µM, SD=25 vs 52 µM, SD=19, p=0.03), although glucocorticoids are known to stimulate IDO, that is increase tryptophan consumption (figure 1). Lower levels of tryptophan were detected in patients without prednisolone compared with controls (52 µM, SD=19 vs 62 µM, SD=14, p=0.006.). There was no difference in tryptophan between patients treated with prednisolone compared with controls. The kynurenine/tryptophan ratio was not significantly different comparing patients with prednisolone (0.018, SD=0.010, n=80) to patients without prednisolone (0.022, SD=0.019, n=52).
Fatigue and depression
In the SLE cohort, 100 of the 132 patients (76%) as well as 10 of the 30 controls (33%) were defined as having severe fatigue. There was a significant difference in quinolinic acid, kynurenine, tryptophan levels and kynurenine/tryptophan ratio when comparing patients with severe fatigue with controls, but no difference was observed when comparing SLE patients with no/limited fatigue with either controls or SLE patients with severe fatigue (table 2).
Fatigue showed an overall weak correlation to quinolinic acid, kynurenine and kynurenine/tryptophan ratio in the SLE cohort (rs =0.21, rs =0.22 and rs =0.25, respectively), but a stronger correlation to these metabolites was observed within a selection of patients that where considered to have severe fatigue (rs =0.28, rs =0.34 and rs =0.25, respectively) (table 3). Index variables with connection to depression and fatigue were investigated (SLAM headache, SLAM fatigue, SLAM cortical dysfunction, SLICC neuropsychiatric, HADS anxiety and HADS depression), but no correlation was found in relation to the metabolites in the kynurenine pathway except weak correlations between kynurenine and SLICC neuropsychiatric damage (rs =0.18, p=0.03) and between tryptophan and SLAM cortical dysfunction (rs =−0.20, p=0.02). HADS anxiety was reported for 61 of 129 patients with SLE and 11 of 30 controls, and HADS depression was reported for 42 of 129 patients and 5 of 30 controls. Additionally, SLEDAI did not correlate with fatigue, and the metabolite levels did not correlate with fatigue among the controls.
Spearman rank correlation between metabolites in the kynurenine pathway and FSS in the entire SLE cohort and in the selection of patients with severe fatigue
Correlation of cytokines to metabolites in the kynurenine pathway
Proinflammatory cytokines associated with SLE and disease activity, that is, IP-10, TNF-α and IL-16, correlated with kynurenine, quinolinic acid and kynurenine/tryptophan ratio, but not with tryptophan, in our study (table 4). MIP-1β correlated weakly with kynurenine/tryptophan ratio (rs =0.24) and IFN-γ correlated only with quinolinic acid levels (rs =0.29).
Spearman rank correlation between metabolites in the kynurenine pathway and selected cytokines
Complementary measurements of the metabolites in the kynurenine pathway
In the GC-MS study, picolinic acid (figure 1) was detected, but patients with SLE and controls did not differ. Tryptophan was detected at lower levels in patients compared with controls in the combined metabolic profile, but the difference reached significance (p=0.02) only in one of the analysed batches of samples. NMR data of urine samples showed significantly (p=0.0041) higher levels of trigonelline (figure 1) in patients with SLE (88.1, SD=85, n=58) compared with control (33.7, SD=42, n=12). No difference was seen in either tryptophan or quinolinic acid urinary levels. Kynurenine was not detected in urine.
Discussion
In this study, we report higher circulating levels of the tryptophan metabolite kynurenine and an increased kynurenine/tryptophan ratio in patients with SLE compared with controls. These findings confirm previous studies reporting changes in the kynurenine pathway in SLE.17–19 We found that levels of tryptophan were numerically lower in SLE, as measured by the LC-MS method, but the difference was not significant, and we can thus not confirm previous studies that reported lower levels of tryptophan in SLE compared with controls. However, using the GC-MS approach we observed lower levels of tryptophan in patients for the combined metabolic profile but did not reach significance for all individual sample batches. When the patients were stratified for disease activity, as measured by two validated indices, we observed lower levels of tryptophan in patients with active SLE compared with controls. In patients with high disease activity, dietary factors might contribute to lower levels of tryptophan. However, the kynurenine/tryptophan ratio is not affected by the diet and therefore a better measure of IDO enzyme activity.
We report higher levels of quinolinic acid in plasma from patients with SLE compared with controls. This finding may be related to an earlier finding of increased levels of quinolinic acid in CSF38 but, to our knowledge, it has not previously been reported in plasma from patients with SLE. Quinolinic acid has been shown to have a wide biological activity and is able to initiate inflammation and can generate reactive oxygen species.39 Quinolinic acid is a de novo precursor to nicotinic acid, which is used in the synthesis of nicotinamide adenine dinucleotide, a coenzyme catalysing redox reactions in the body.13 In urine, a methylated form of nicotinic acid, that is, trigonelline, can be found and a change in the excretion of trigonelline can be an indicator of niacin metabolism, a process that can be altered by tryptophan metabolism and also by dietary sources.40 We detected higher levels of urinary trigonelline in SLE compared with controls. We suggest that high urinary trigonelline indicates enhanced activity of the kynurenine pathway and possible shunting towards quinolinic acid. The majority of the patients were on prednisolone treatment, and it is known that glucocorticoids upregulate the expression of TDO that metabolises tryptophan,41 and we may thus expect increased kynurenine/tryptophan ratio due to treatment. However, there was no significant difference in kynurenine/tryptophan ratio comparing patients with glucocorticoid treatment and untreated patients. IDO, the other enzyme converting tryptophan to kynurenine, can be induced by proinflammatory stimuli such as IFN-γ, TNF-α and LPS,42 and we observed that the kynurenine/tryptophan ratio correlated positively to TNF-α (rs =0.48). It has been suggested that IDO-dependent immunosuppressive mechanisms are activated in SLE.19 Interferons and proinflammatory cytokines such as TNF-α, which are associated with SLE disease activity, may cause increased IDO enzyme activity and be part of the pathogenesis of SLE. Whether the metabolites in the kynurenic pathway have specific functions or contribute to disease pathogenesis has not been evaluated in this work. In the aim to suppress IDO activity, niacin (vitamin B12) supplementation9 and IDO blockers43 might be interesting treatment perspectives in SLE.
Depression, other cognitive issues or indexes for disease activity did not show any correlation to quinolinic acid, kynurenine, tryptophan or kynurenine/tryptophan ratio; however, a weak correlation to fatigue was observed. We suggest that these results indicate that the kynurenine pathway is mainly important in SLE-related fatigue. It has previously been suggested that activation of the IDO pathway may be relatively unique to inflammation-induced depression44 and has also been suggested as a link between inflammatory cytokines and cancer-related fatigue.45 The levels of fatigue was slightly higher among our controls than the values originally reported as normal for healthy adults,25 but they were comparable with controls in more recent European studies.25 28 The choice of questionnaires to capture patient-reported outcomes, such as fatigue, also influences the results. However, several instruments that measure fatigue showed reliable results when exploring clinically important difference, as defined by patients.46 The fatigue questionnaire used in this study, FSS, is widely used and recommended in SLE.47 In this study, a majority of the patients reported FSS ≥4. Fewer patients reported high levels of anxiety or depression (HADS ≥7), which indicate symptoms of mental disorder.
In conclusion, we confirm previous studies that the kynurenine pathway is altered in patients with SLE compared with controls. The metabolites in the kynurenine pathway have many different functions, and an increased activity of the pathway could be involved in the pathogenesis of SLE. The increased IDO activity could be a result of proinflammatory cytokines. We demonstrate that SLE-related fatigue, as captured by FSS, correlated weakly to the tryptophan metabolites kynurenine and quinolinic acid, while depression (HADS) did not. We believe that these observations may be of future clinical relevance, and it is relevant to investigate if drugs targeting IDO are effective treatments in SLE.
Acknowledgments
We would like to thank the patients and controls for participating in the study. Eva Jemseby for collecting all the blood samples and coordinating nurses Sonia Möller and Birgitta Mannerstedt Fogelfors for administration of patient questionnaires.
References
Footnotes
KÅ and SP contributed equally.
Contributors KÅ: statistical analysis and manuscript writing. SP: FSS data collection and analysis and manuscript writing. SS: LC-MS analysis of metabolites in the kynurenine pathway. IS: GC-MS analysis and manuscript writing. MH: NMR analysis and manuscript writing. SE: cytokine measurements and manuscript writing. JT and P-PJ: manuscript writing/approval. IG and ES: SLE cohort responsible and manuscript writing. HI: study design, statistical analyses and manuscript writing.
Funding This study was supported by the AstraZeneca–Karolinska Institutet Joint Research Program in Translational Science, Swedish Research Council, Stockholm County Council (ALF), Swedish Heart-Lung foundation, King Gustaf Vs 80th Birthday Fund, Swedish Rheumatism Association, Swedish Society of Medicine and Karolinska Institutet’s Foundations.
Competing interests SE is employed by AstraZeneca.
Patient consent Obtained.
Ethics approval The local research ethics committee approved the study.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.