Article Text
Abstract
Objectives In lupus nephritis the pathological diagnosis from tissue retrieved during kidney biopsy drives treatment and management. Despite recent approval of new drugs, complete remission rates remain well under aspirational levels, necessitating identification of new therapeutic targets by greater dissection of the pathways to tissue inflammation and injury. This study assessed the safety of kidney biopsies in patients with SLE enrolled in the Accelerating Medicines Partnership, a consortium formed to molecularly deconstruct nephritis.
Methods 475 patients with SLE across 15 clinical sites in the USA consented to obtain tissue for research purposes during a clinically indicated kidney biopsy. Adverse events (AEs) were documented for 30 days following the procedure and were determined to be related or unrelated by all site investigators. Serious AEs were defined according to the National Institutes of Health reporting guidelines.
Results 34 patients (7.2%) experienced a procedure-related AE: 30 with haematoma, 2 with jets, 1 with pain and 1 with an arteriovenous fistula. Eighteen (3.8%) experienced a serious AE requiring hospitalisation; four patients (0.8%) required a blood transfusion related to the kidney biopsy. At one site where the number of cores retrieved during the biopsy was recorded, the mean was 3.4 for those who experienced a related AE (n=9) and 3.07 for those who did not experience any AE (n=140). All related AEs resolved.
Conclusions Procurement of research tissue should be considered feasible, accompanied by a complication risk likely no greater than that incurred for standard clinical purposes. In the quest for targeted treatments personalised based on molecular findings, enhanced diagnostics beyond histology will likely be required.
- lupus nephritis
- lupus erythematosus
- systemic
- autoimmunity
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known about this subject?
A clinically indicated kidney biopsy is thought to be a relatively safe procedure and plays an integral role in the diagnosis of lupus nephritis.
Retrieval of tissue for research is a limiting factor for studies seeking to mechanistically deconstruct lupus nephritis at the molecular level.
What does this study add?
The Accelerating Medicines Partnership lupus nephritis experience showed that retrieving additional tissue for research during a clinically indicated kidney biopsy is safe and feasible, with the most common adverse events being bleed-related, occurring in 6.7% of patients, all of which resolved.
How might this impact on clinical practice or future developments?
This study has shown that procurement of additional tissue beyond traditional histology for lupus nephritis may be considered as part of standard of care in the era of personalised molecular medicine and for future studies seeking to elucidate therapeutic targets and molecular mechanisms underlying lupus nephritis.
Introduction
SLE is a multiorgan autoimmune disorder primarily affecting women of childbearing age.1 Lupus nephritis (LN), which affects nearly 60% of patients during the course of their disease, carries the highest standardised mortality ratio in SLE.2–4 While clinical abnormalities such as elevated proteinuria and active urine sediment signal kidney inflammation, percutaneous kidney biopsy is traditionally done to confirm the diagnosis and formulate the optimal treatment plan based on the International Society of Nephrology (ISN) class and the National Institutes of Health (NIH) Activity Index.5–7 Following a drought in new drug development for several decades, demonstrated efficacy in two registrational Phase III randomised controlled trials supported the approval of both belimumab and voclosporin for LN.8 9 However, the ‘holy grail’ of identifying new and effective targeted therapies that induce complete and durable responses remains a high priority, as nearly 60% of patients did not achieve the primary kidney endpoints in either trial.
Accordingly, continued insights into the pathogenesis are critical. Indeed ‘tissue is the issue’, and access to kidney biopsies for research purposes should be highly informative. Detailed characterisation of the cell types and expression profiles accompanying histological assignments will enable investigators to link phenotype (as measured by clinical characteristics and response to therapy) to ‘biotype’ and, by so doing, point the way to the development of new, more effective and personalised therapies. Access to diseased tissue may address the striking ethnic and racial health disparities that characterise SLE10 by determining the extent to which distinct molecular pathways account for the increased frequency and severity of LN in certain groups.
Initiated to molecularly deconstruct LN, the Accelerating Medicines Partnership (AMP) is a public–private consortium involving the NIH, pharmaceutical companies and lupus investigators across 15 clinical sites collecting urine, blood, skin biopsies and kidney biopsies from patients with LN.11 The availability of these biospecimens has already yielded informative results, including the demonstration that a type I interferon response signature and fibrotic pathways associated with 1-year response/non-response.12 13 Kidney transcriptomics also revealed evidence of local activation of B cells which correlated with an age-associated B cell signature and evidence of progressive stages of monocyte differentiation.12 However, all patients enrolled in AMP consented to the donation of kidney tissue retrieved during clinically indicated biopsies, potentially incurring an increased risk of an adverse event (AE) given the additional tissue harvested for research purposes. All AEs occurring across the entirety of the clinical sites during or within 30 days of the biopsy were reported with the goal of establishing the safety of this approach, not only for future research studies, but for consideration as an addition to standard of care.
Methods
Patients undergoing a clinically indicated kidney biopsy to evaluate proteinuria (defined for inclusion in AMP as a urine protein creatinine ratio >0.5) at the direction of the treating rheumatologist or nephrologist were approached and recruited for this study if they were over the age of 16 and fulfilled sufficient criteria for the diagnosis of SLE based on the revised American College of Rheumatology or the Systemic Lupus Erythematosus International Collaborating Clinics classification criteria.14 15 Patients with a history of kidney transplant, recent use of rituximab within 6 months of biopsy (as it might influence the transcriptome) or if pregnant were excluded. All patients provided written informed consent. AMP comprised three phases: 0 for technical development, 1 for short-term evaluation of patients and 2 for a longitudinal evaluation through 52 weeks. Biopsies done in all phases are included in this safety report.
Across all sites, ultrasound-guided or CT-guided kidney biopsies were performed by interventional radiologists or nephrologists using a 16-gauge to 18-gauge needle. Based on the guidelines established by the Society of Interventional Radiology, a platelet count greater than 50 cells x 109/L was required prior to biopsy.16 Even higher thresholds were applied, depending on the specific AMP site. In the majority of cases, research tissue was obtained at the time of kidney biopsy as an additional core taken solely for research purposes, or in less than 15% by immediately obtaining a piece of a core with sufficient glomeruli that was not required solely for clinical diagnosis. Clinical tissue was reviewed by site pathologists, who assigned the ISN class and activity and chronicity indices.5–7 All research tissue obtained was processed immediately and maintained in Cryostor for downstream transcriptomic analysis as previously described.12 13
AEs, defined as any occurrence or worsening of an undesirable or unintended sign, symptom, laboratory finding or disease within 30 days of biopsy, were documented and reported, including length, severity, type and resolution. As per standard definition, an AE was considered ‘serious’ if it resulted in any of the following outcomes: death, life-threatening event, inpatient hospitalisation or prolongation of existing hospitalisation, persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions, requiring intervention to prevent permanent impairment or damage, or an important medical event based on appropriate medical judgement. Site-specific kidney biopsy monitoring protocols were followed with postprocedure laboratory testing often only obtained in cases where patients were symptomatic. AEs were defined by the site investigator. Detailed reports were provided to a central adjudicator.
Results are represented as N and % for categorical variables and median (IQR) for continuous variables. Means are also included for the number of passes. Sample size is listed for variables where it differed from the overall sample. For variables listed in table 1, a Student’s t-test or Wilcoxon’s test were used to compare continuous variables and Pearson’s chi-squared or Fisher’s exact were used to compare categorical variables where appropriate. No calculated p-values were less than 0.05 and were therefore omitted from table 1.
Demographics and characteristics of patients undergoing clinically indicated kidney biopsy
Results
Between 2014 and 2020, 475 patients with SLE underwent a kidney biopsy for AMP. As detailed in table 1, the cohort comprised predominantly women (82%) and was representative of the racial and ethnic backgrounds known to have a higher incidence of LN in the USA, such as those identifying as black, Hispanic and Asian.4 Overall, 29% had proliferative nephritis, 22% membranous, 26% mixed, 8% mesangial, 4% advanced fibrotic and 11% were non-LN or had insufficient tissue for diagnosis.
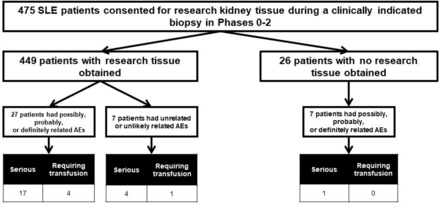
As shown in figure 1, research tissue was not retrieved during the biopsy for 26 (5%) of the 475 patients. Seven of these experienced a related AE which prevented research tissue collection. Of the 449 patients in whom research tissue was collected, 27 (6%) experienced a related AE and 7 (1.5%) experienced an unrelated AE. Thus, overall as detailed further in table 1, 41 (8.6%) of the 475 patients experienced any AE, either related or unrelated. Of the total AEs, 34 had an AE deemed possibly, probably or definitely related to the biopsy procedure, resulting in an overall related AE rate of 7.2%. The distribution of biopsy class, activity index, chronicity index and first or repeat biopsy did not differ for those with or without an AE related to the biopsy procedure (table 1). There were no statistically significant differences among any of the variables listed in table 1 between patients who experienced an AE and those who did not.
Flow diagram of adverse events (AEs) by relationship and seriousness.
As expected, the most common related AEs experienced after kidney biopsy were bleed-related complications, either a jet at the time of biopsy, haematoma at the biopsy site and/or a decrease in haemoglobin, occurring in 32 patients (6.7%) (table 2). Of the patients with related AEs, 18 (3.8%) patients of 475 had an AE deemed to be serious. All were bleed-related complications and required hospitalisation. Overall, only 4 (0.84%) patients of 475 required blood transfusion related to the kidney biopsy. All related AEs resolved within 15 days. Of note, in two subjects, more than one AE was reported. One patient had haematoma, haemoglobin decrease and two episodes of flank pain reported separately, but all deemed probably related to the study procedure and counted as one related AE. Another subject had two AEs reported: one for haemoglobin decrease (related) and one for shortness of breath (unrelated). In seven patients, the AEs were deemed unrelated or unlikely to be related to the study procedure. These events included various types of body pain aside from flank pain, fall, cardiac arrest, sepsis requiring a transfusion without any kidney haematoma and dyspnoea. Four of these unrelated AEs were categorised as serious AEs and required hospitalisation. The unrelated cardiac arrest occurred 19 days after kidney biopsy and was fatal. All other unrelated AEs and serious AEs resolved (table 2).
Kidney biopsy complications
A subgroup analysis was done to evaluate whether the number of passes obtained influenced the frequency of a related AE. This information was captured only at the New York University (NYU) site (n=153 patients) as the number of passes was specifically recorded in the procedural records of the interventional radiologists or nephrologists. The mean number of passes for those with related AEs was 3.44 and for unrelated AEs was 3.07 (table 1). For two patients requiring a transfusion, the number of passes was 3 and 4. For two patients in whom the related AEs were not considered serious, the number of passes was 3 and 4.
Discussion
This is the first study in LN, across multiple US sites, to assess the risk of kidney biopsy during which tissue was obtained for research as part of the consent at the time of a clinically indicated procedure. The data support that this procurement is feasible with high patient acceptance and that the rate of serious AE is comparable with published data for clinically obtained kidney biopsies.17–22 Overall, 7.2% of the 475 patients with SLE experienced an AE attributable to the biopsy procedure, with an overall related serious AE rate of 3.8%. As expected, bleed-related complications, such as haematoma and haemoglobin decrease, were the most commonly reported complications, with less than 1% requiring a transfusion. The risk of complications was not associated with biopsy class, activity index, chronicity index, first or repeat biopsy, or number of cores obtained. Seven patients experienced an AE not considered related to the procedure.
Recent publications have shown that the number of needle passes does not correlate with risk of kidney biopsy complications.18 23 24 This is in agreement with the AMP experience and supports that obtaining research tissue is not associated with a higher risk than the clinically indicated procurement of kidney tissue for diagnosis. In the only study to show a borderline statistically significant increase in complication risk with the number of passes, when controlling for potential confounders, the bleeding risk was only greater when the number of passes exceeded 5.25
The safety of kidney biopsies done solely for clinical purposes has been previously reported and rates differ depending on the underlying disease. In large single-centre studies of kidney biopsies done for varying diseases, complication rates related to the procedure ranged from 14% to 17%, whereas studies of patients with autoimmune diseases (or specifically SLE) ranged from 4% to 11%.17–22 A recent meta-analysis has shown that in all studies bleed-related complications were the AEs most commonly observed.26 There are few studies that have extensively reported on the safety of a clinically indicated kidney biopsy specifically for suspected LN. In a retrospective study of 219 patients with SLE at Johns Hopkins undergoing a clinically indicated kidney biopsy, the overall incidence of bleeding was 10.5%, with 2.7% considered major requiring intervention with a transfusion, interventional radiology or surgery; haemodynamic instability requiring transfer to a higher level of care or need for vasopressors; or death.20 Comparatively, patients in AMP had fewer such events despite research tissue being obtained. In the Hopkins study, patients with a platelet count <150 cells x 109/L were 30 times more likely to experience a major complication (p=0.002). Other candidate predictors, including glucocorticoid exposure, kidney function, haematocrit and histopathology, were not significant. In our study, no clinical variables could distinguish patients who experienced an AE from those who did not.
There is an increasing interest in performing serial biopsies on patients with LN, which has thus far not been associated with increased risk of AEs.27 In support of this, in our study 9% of patients at first biopsy experienced a related AE, whereas 7% of patients undergoing a repeat biopsy had a related AE. However, concern regarding the safety of a second biopsy and obtaining research tissue might relate to the implication that AEs are more common with progressive fibrosis. The median chronicity index was 5 in those with an AE vs 3 in those without an AE, which did not reach statistical significance, but it is acknowledged that the study may be underpowered given few AEs.
A recently published study has assessed the safety of obtaining an additional research core from patients with diabetes nephropathy during a clinically indicated biopsy.28 In this smaller study of 160 patients, Hogan et al28 reported a 7% biopsy-related complication rate, which is similar to what has been observed in the AMP study. This suggests that the safety of obtaining additional kidney tissue is not limited to LN and reinforces that this methodology has the potential to maximise specimens available for discovery analyses in a wide range of kidney diseases.
Acknowledged limitations in the AMP study include the absence of uniformity of the procedure, variable postprocedure monitoring and management, incomplete information on the number of passes done at each biopsy, and the inclusion of a minority of cases where tissue was obtained from the same core used for clinical determination. Blood pressure and data on managing anticoagulation prior to the biopsy procedure were not collected, and it is unknown whether these variables impacted bleeding risk. Based on AMP, procurement of research tissue is feasible with no apparent increased complication risk over that for standard clinical purposes. Thus, a research core can be considered for future studies to advance discovery of new therapeutic targets and prognostic indicators in LN. In moving towards personalised medicine, results from these studies will be critically important for the advancement of clinical care as we reach beyond traditional histology.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
Each site’s institutional review board approved its participation under the respective protocols in adherence to the principles of the Declaration of Helsinki: Cedars: IRB #Pro00050132; Cincinnati: IRB #2016-1246; Einstein: IRB #2014-4079; Johns Hopkins: NA_00039294; Michigan: IRB #HUM00093859; MUSC: IRB #Pro00067258; Northwell: IRB #17-0572; NYU: IRB#14-01663; Rochester: RSRB #55411; Temple: #23353; Texas Tech: IRB #E17093; UC sites, including UCSF, UCLA and UCSD: IRB #17-21618; UNC: IRB #15-2847; UT Health: IRB #HSC-MS-17-0572.
Acknowledgments
The authors would like to express their gratitude to all the patients who participated in this study. They would also like to thank Benjamin Wainwright for his assistance with the manuscript.
References
Footnotes
MAP and JPB are joint senior authors.
Contributors The study was conceptualised by KD, PC, AF, DW, JAJ, CP, BD, AD, DF, JM-T, MGA, DR, WA, HMB, PI, RAF, MD'E, DLK, KK, MAP and JPB. Data curation was completed by KD, PC, AF, JL, DW, JAJ, CP, KH, WA, PI, MW, SC, FP-S, RAF, MD'E, DLK, MAP and JPB. Formal analysis was completed by KD, PC, AF, JL, DW, JAJ, CP, MAP and JPB. Funding was acquired by AF, DW, JAJ, CP, MAP and JPB. Investigation was performed by KD, PC, AF, DW, JAJ, CP, BD, AD, DF, JM-T, MGA, DR, WA, HMB, PI, CCB, KC, MI, MW, MAP and JPB. Methodology was designed by KD, PC, AF, JL, DW, JAJ, CP, MAP and JPB. Project administration was performed by KD, PC, AF, DW, WA, MAP and JPB. Resources were provided by DW, CP, BD, AD, DF, JM-T, MGA, HMB, PI, MW, RAF, MD'E, DLK, KK, JA, MW, MAP and JPB. Software analysis was performed by JL. Supervision was provided by DW, MAP and JPB. Validation was performed by AF. The original draft of the manuscript was written by KD, PC, MAP and JPB. All authors assisted with review and editing of the final manuscript.
Funding This work was supported by the Accelerating Medicines Partnership (AMP) in Rheumatoid Arthritis and Lupus Network. AMP is a public-private partnership (AbbVie Inc., Arthritis Foundation, Bristol-Myers Squibb Company, Foundation for the National Institutes of Health, GlaxoSmithKline, Janssen Research and Development, LLC, Lupus Foundation of America, Lupus Research Alliance, Merck Sharp & Dohme Corp., National Institute of Allergy and Infectious Diseases, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Pfizer Inc., Rheumatology Research Foundation, Sanofi and Takeda Pharmaceuticals International, Inc.) created to develop new ways of identifying and validating promising biological targets for diagnostics and drug development Funding was provided through grants from the National Institutes of Health (UH2-AR067676, UH2-AR067677, UH2-AR067679, UH2-AR067681, UH2-AR067685, UH2- AR067688, UH2-AR067689, UH2-AR067690, UH2-AR067691, UH2-AR067694, and UM2- AR067678).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.