Abstract
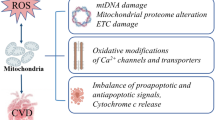
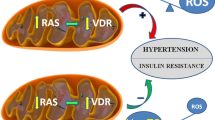
The metabolic syndrome is a constellation of metabolic disorders including obesity, hypertension, and insulin resistance, components which are risk factors for the development of diabetes, hypertension, cardiovascular, and renal disease. Pathophysiological abnormalities that contribute to the development of the metabolic syndrome include impaired mitochondrial oxidative phosphorylation and mitochondrial biogenesis, dampened insulin metabolic signaling, endothelial dysfunction, and associated myocardial functional abnormalities. Recent evidence suggests that impaired myocardial mitochondrial biogenesis, fatty acid metabolism, and antioxidant defense mechanisms lead to diminished cardiac substrate flexibility, decreased cardiac energetic efficiency, and diastolic dysfunction. In addition, enhanced activation of the renin–angiotensin–aldosterone system and associated increases in oxidative stress can lead to mitochondrial apoptosis and degradation, altered bioenergetics, and accumulation of lipids in the heart. In addition to impairments in metabolic signaling and oxidative stress, genetic and environmental factors, aging, and hyperglycemia all contribute to reduced mitochondrial biogenesis and mitochondrial dysfunction. These mitochondrial abnormalities can predispose a metabolic cardiomyopathy characterized by diastolic dysfunction. Mitochondrial dysfunction and resulting lipid accumulation in skeletal muscle, liver, and pancreas also impede insulin metabolic signaling and glucose metabolism, ultimately leading to a further increase in mitochondrial dysfunction. Interventions to improve mitochondrial function have been shown to correct insulin metabolic signaling and other metabolic and cardiovascular abnormalities. This review explores mechanisms of mitochondrial dysfunction with a focus on impaired oxidative phosphorylation and mitochondrial biogenesis in the pathophysiology of metabolic heart disease.



Similar content being viewed by others
References
Sullivan PW, Ghushchyan V, Wyatt HR, Wu EQ, Hill JO (2007) Impact of cardiometabolic risk factor clusters on health-related quality of life in the U.S. Obesity (Silver Spring) 15:511–521
Cannon CP, Kumar A (2009) Treatment of overweight and obesity: lifestyle, pharmacologic, and surgical options. Clin Cornerstone 9:55–68, discussion 69–71
Sowers JR (2007) Metabolic risk factors and renal disease. Kidney Int 71:719–720
Whaley-Connell A, Sowers JR (2009) Hypertension and insulin resistance. Hypertension 54:462–464
Smith SC Jr (2007) Multiple risk factors for cardiovascular disease and diabetes mellitus. Am J Med 120:S3–S11
Cooper SA, Whaley-Connell A, Habibi J et al (2007) Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol 293:H2009–H2023
Kim JA, Wei Y, Sowers JR (2008) Role of mitochondrial dysfunction in insulin resistance. Circ Res 102:401–414
Sowers JR, Whaley-Connell A, Epstein M (2009) The emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med 150:776–785
Bassuk SS, Manson JE (2005) Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol 99:1193–1204
Zhou X, Ma L, Habibi J, Whaley-Connell A, Hayden MR, Tilmon RD, Brown AN, Kim JA, Demarco VG, Sowers JR (2010) Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension 55(4):880–888
Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J (2003) AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular moycytes: role of the AT1 receptor and NADPH oxidase. Hypertension 42:206–212
Ren J, Kelley RO (2009) Cardiac health in women with metabolic syndrome: clinical aspects and pathophysiology. Obesity (Silver Spring) 17:1114–1123
Finck BN, Kelly DP (2007) Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation 115(19):2540–2548
Ren J, Bode AM (2000) Altered cardiac excitation-contraction coupling in ventricular myocytes from spontaneously diabetic BB rats. Am J Physiol Heart Circ Physiol 279:H238–H244
Wold LE, Ceylan-Isik AF, Ren J (2005) Oxidative stress and stress signaling: menace of diabetic cardiomyopathy. Acta Pharmacol Sin 26:908–917
Boudina S, Abel ED (2007) Diabetic cardiomyopathy revisited. Circulation 115:3213–3223
Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H et al (2002) Defective intracellular Ca(2+) signaling contributes to cardiomyopathy in type 1 diabetic rats. Am J Physiol Heart Circ Physiol 283:H1398–H1408
Dong F, Li Q, Sreejayan N, Nunn JM, Ren J (2007) Metallothionein prevents high-fat diet induced cardiac contractile dysfunction: role of peroxisome proliferator activated receptor gamma coactivator 1alpha and mitochondrial biogenesis. Diabetes 56:2201–2212
Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S et al (2006) Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes 55:608–615
Flagg TP, Cazorla O, Remedi MS, Haim TE, Tones MA et al (2009) Ca2 + -independent alterations in diastolic sarcomere length and relaxation kinetics in a mouse model of lipotoxic diabetic cardiomyopathy. Circ Res 104:95–103
Zhang L, Cannell MB, Phillips AR, Cooper GJ, Ward ML (2008) Altered calcium homeostasis does not explain the contractile deficit of diabetic cardiomyopathy. Diabetes 57:2158–2166
Lowell BB, Shulman GI (2005) Mitochondrial dysfunction and type 2 diabetes. Science 307:384–387
Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R et al (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S et al (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273
Davidson SM, Duchen MR (2007) Endothelial mitochondria: contributing to vascular function and disease. Circ Res 100:1128–1141
Zhang DX, Gutterman DD (2007) Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292:H2023–H2031
Nishio Y, Kanazawa A, Nagai Y, Inagaki H, Kashiwagi A (2004) Regulation and role of the mitochondrial transcription factors in the diabetic heart. Ann NY Acad Sci 1011:78–85
Nisoli E, Clementi E, Carruba MO, Moncada S (2007) Defective mitochondrial biogenesis: a hallmark of the high cardiovascular risk in the metabolic syndrome? Circ Res 100:795–806
Lopaschuk GD, Spafford M (1989) Response of isolated working hearts to fatty acids and carnitine palmitoyltransferase I inhibition during reduction of coronary flow in acutely and chronically diabetic rats. Circ Res 65:378–387
Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ (2009) PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol 46:201–212
de Cavanagh EM, Toblli JE, Ferder L, Piotrkowski B, Stella I, Inserra F (2006) Renal mitochondrial dysfunction in spontaneously hypertensive rats is attenuated by losartan but not by amlodipine. Am J Physiol Regul Integr Comp Physiol 290(6):R1616–R1625
Doughan AK, Harrison DG, Dikalov SI (2008) Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102:488–496
Mitsuishi M, Miyashita K, Muraki A, Itoh H (2009) Angiotensin II reduces mitochondrial content in skeletal muscle and affects glycemic control. Diabetes 58:710–717
Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR et al (2007) Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology 148:3773–3780
Whaley-Connell A, Habibi J, Cooper SA, Demarco VG, Hayden MR et al (2008) Effect of renin inhibition and AT1R blockade on myocardial remodeling in the transgenic Ren2 rat. Am J Physiol Endocrinol Metab 295:E103–E109
Kelley DE, He J, Menshikova EV, Ritov VB (2002) Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950
Morino K, Petersen KF, Dufour S, Befroy D, Frattini J et al (2005) Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115:3587–3593
Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH et al (2005) Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54:8–14
Handschin C, Spiegelman BM (2008) The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 454:463–469
Reznick RM, Shulman GI (2006) The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol 574:33–39
Ling C, Poulsen P, Carlsson E, Ridderstrale M, Almgren P et al (2004) Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest 114:1518–1526
St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R et al (2003) Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem 278:26597–26603
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G et al (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124
Arany Z, He H, Lin J, Hoyer K, Handschin C et al (2005) Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab 1:259–271
Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP (2007) Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation 115:909–917
Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R et al (2007) Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50:790–796
Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L et al (2004) Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci USA 101:16507–16512
Ceylan-Isik AF, Guo KK, Carlson EC, Privratsky JR, Liao SJ et al (2009) Metallothionein abrogates GTP cyclohydrolase I inhibition-induced cardiac contractile and morphological defects: role of mitochondrial biogenesis. Hypertension 53:1023–1031
Czubryt MP, McAnally J, Fishman GI, Olson EN (2003) Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci USA 100:1711–1716
Russell LK, Finck BN, Kelly DP (2005) Mouse models of mitochondrial dysfunction and heart failure. J Mol Cell Cardiol 38:81–91
Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P et al (2001) Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab 281:E1340–E1346
Turdi S, Fan X, Li J, Zhao J, Huff AF et al (2010) AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell 9:592–606
Baron SJ, Li J, Russell RR 3rd, Neumann D, Miller EJ et al (2005) Dual mechanisms regulating AMPK kinase action in the ischemic heart. Circ Res 96:337–345
Capano M, Crompton M (2006) Bax translocates to mitochondria of heart cells during simulated ischaemia: involvement of AMP-activated and p38 mitogen-activated protein kinases. Biochem J 395:57–64
Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T et al (2005) Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res 97:837–844
Shigenaga MK, Hagen TM, Ames BN (1994) Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA 91:10771–10778
Brandes RP (2005) Triggering mitochondrial radical release: a new function for NADPH oxidases. Hypertension 45(5):847–848
Wei Y, Whaley-Connell A, Sowers JR et al (2009) Mineralocorticoid receptor antagonism attenuates vascular apoptosis and injury via rescuing Akt activation. Hypertension 53(2):158–165
Fukai T (2009) Mitochondrial thioredoxin: novel regulator for NADPH oxidase and angiotensin II-induced hypertension. Hypertension 54(2):224–225
Sebastiani M, Giordano C, Nediani C, Travaglini C, Borchi E et al (2007) Induction of mitochondrial biogenesis is a maladaptive mechanism in mitochondrial cardiomyopathies. J Am Coll Cardiol 50:1362–1369
Yu T, Robotham JL, Yoon Y (2006) Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA 103:2653–2658
Makino A, Scott BT, Dillmann WH (2010) Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 53:1783–1794
Williamson CL, Dabkowski ER, Baseler WA, Croston TL, Alway SE, Hollander JM (2010) Enhanced apoptotic propensity in diabetic cardiac mitochondria: influence of subcellular spatial location. Am J Physiol Heart Circ Physiol 298:H633–H642
Acknowledgements
Research included in this review is supported by NIH R01HL073101, VA Merit (JRS), and CDA-2 Dept of Veterans Affairs (AWC). The authors thank Brenda Hunter for her assistance in preparing this manuscript.
Conflict of interest statement
The authors declare no conflict of interests related to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, J., Pulakat, L., Whaley-Connell, A. et al. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med 88, 993–1001 (2010). https://doi.org/10.1007/s00109-010-0663-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-010-0663-9