Abstract
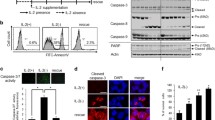
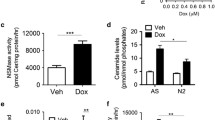
Lactosylceramide (LacCer) is a member of the glycosphingolipid family which has been recently recognized as a signaling intermediate in the regulation of cell proliferation and cell adhesion. In this paper, we present our studies pointing to a potential role of LacCer in inducing apoptosis. In our studies we employed a human osteosarcoma cell line MG-63 (wild type, WT) and a neutral sphingomyelinase (N-SMase) deficient cell line CC derived from MG-63 (mutant) cells. We observed that WT cells were highly sensitive to tumor necrosis factor-α (TNF-α), ceramide and LacCer-induced apoptosis. In contrast, the mutant cells were insensitive to TNF-α-induced apoptosis as they did not generate ceramide and LacCer. However, the exogenous supply of ceramide and/or LacCer rendered the mutant cells apoptotic. Interestingly, preincubation of cells with D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (D-PDMP), an inhibitor of glucosylceramide synthase and lactosylceramide synthase, abrogated ceramide-induced apoptosis but not LacCer-induced apoptosis in both WT cells and the mutant cells. Moreover, TNF-α and LacCer-induced apoptosis required the generation of reactive oxygen species (ROS) in WT cells. However, since mutant cells did not produce significant amounts of LacCer and ROS in response to TNF-α treatment they are insensitive to TNF-α-induced apoptosis. In summary, our studies suggest that TNF-α-induced N-SMase activation and production of ceramide is required to activate the apoptosis pathway in human osteosarcoma cells. But it is not sufficient to induce apoptosis. Rather, the conversion of ceramide to LacCer and ROS generation are critical for apoptosis.
Similar content being viewed by others
Abbreviations
- LacCer:
-
LactosylCeramide
- TNF-α:
-
Tumor Necrosis Factor-α
- N-SMase:
-
Neutral Sphingomyelinase
- D-PDMP:
-
D-threo-1-Phenyl-2-Decanoylamino-3-Morpholino-1-Propanol
- ROS:
-
Reactive Oxygen Species
- MnSOD:
-
Manganese Superoxide Dismutase
- NAC:
-
N-acetyl-L-cysteine
- PBS:
-
Phosphate-Buffered Saline
- DTT:
-
Dithiothreitol
- ATP:
-
Adenosine Tris-Phosphate
- DAPI:
-
4’,6-Diamidine-2’-phenylindole dihydrochloride
- DCFH-DA:
-
diachlorofluorescein diacetate
- TNFR1:
-
TNF-α receptor 1
- SM:
-
sphingomyelin; Wild type
- WT:
-
human osteosarcoma cell line MG-63
- Mutant:
-
N-SMase deficient osteosarcoma cell line CC
References
Hakomori, S.: Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Ann. Rev. Biochem. 50, 733–64 (1981)
Karlsson, K.A.: Animal glycosphingolipids as membrane attachment sites for bacteria. Ann. Rev. Biochem. 58, 309–350 (1989)
Lavie, Y., Cao, H., Bursten, S.L., Giuliano, A.E., Cabot, M.C.: Accumulation of glucosylceramides in multidrug-resistant cancer cells. J. Biol. Chem. 271, 19530–19536 (1996)
Lavie, Y., Cao, H., Volner, A., Lucci, A., Han, T., Geffen, V., Giuliano, A.E., Cabot, M.C.: Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. J. Biol. Chem. 272, 1682–1687 (1997)
Chatterjee, S.: Lactosylceramide stimulates aortic smooth muscle cell proliferation. Biochem. Biophys. Res. Commun. 181, 554–561 (1991)
Bhunia, A.K., Han, H., Snowden, A., Chatterjee, S.: Redox-regulated signaling by lactosylceramide in the proliferation of human aortic smooth muscle cells. J. Biol. Chem. 272, 15642–15649 (1997)
Bhunia, A.K., Han, H., Snowden, A., Chatterjee, S.: Lactosylceramide stimulates Ras-GTP loading, kinases (MEK, Raf), p44 mitogen-activated protein kinase, and c-fos expression in human aortic smooth muscle cells. J. Biol. Chem. 271, 10660–10666 (1996)
Obeid, L.M., Linardic, C.M., Karolak, L.A., Hannum, Y.A.: Programmed cell death induced by ceramide. Science 259, 1769–1771 (1993)
Chatterjee, S.: Sphingolipids in atherosclerosis and vascular biology. Art. Thromb. Vasc. Biol. 18, 1523–1533 (1998)
Hakomori, S.I.: In Spingolipid Chemistry, edited by Kanfer JN, Hakomori SI (Plenum Press, New York, 1983), pp. 52–53.
Kakugawa, Y., Wada, T., Yamaguchi, K., Yamanami, H., Ouchi, K., Sato, I., Miyagi, T.: Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proc. Natl. Acad. Sci. USA 99, 10718–10723 (2002)
Yeh, L.H., Kinsey, A.M., Chatterjee, S., Alevriadou, B.R.: Lactosylceramide mediates shear-induced endothelial superoxide production and intercellular adhesion molecule-1 expression. J. Vasc. Res. 38, 551–559 (2001)
Iwamoto, T., Fukumoto, S., Kanaoka, K., Sakai, E., Shibata, M., Fukumoto, E., Inokuchi, J.J., Takamiya, K., Furukawa, K., Furukawa, K., Kato, Y., Mizuno, A.: Lactosylceramide is essential for the osteoclastogenesis mediated by macrophage-colony-stimulating factor and receptor activator of nuclear factor-kappa B ligand. J. Biol. Chem. 276, 46031–46038 (2001)
Bhunia, A.K., Arai, T., Bulkley, G., Chatterjee, S.: Lactosylceramide mediates tumor necrosis factor-alpha-induced intercellular adhesion molecule-1 (ICAM-1) expression and the adhesion of neutrophil in human umbilical vein endothelial cells. J. Biol. Chem. 273, 34349–34357 (1998)
Arai, T., Bhunia, A.K., Chatterjee, S., Bulkley, G.B.: Lactosylceramide stimulates human neutrophils to upregulate Mac-1, adhere to endothelium, and generate reactive oxygen metabolites in vitro. Circ. Res. 82, 540–547 (1998)
Polyak, K., Xia, Y., Zweier, J.L., Kinzler, K.W., Voglestein, B.: A model for p53-induced apoptosis. Nature 389, 300–305 (1997)
Cai, J., Jones, D.P.: Mitochondrial redox signaling during apoptosis. J. Bioenerg. Biomembr. 31, 327–334 (1999)
Gardner, A.M., Xu, F.H., Fady, C., Jacoby, F.J., Duffey, D.C., Tu, Y., Lichtenstein, A.: Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free. Radical. Biol. Med. 22, 73–83 (1997)
Sato, N., Iwata, S., Nakamura, K., Hori, T., Mori, K., Yodoi, J.: Thiol-mediated redox regulation of apoptosis. Possible roles of cellular thiols other than glutathione in T cell apoptosis. J. Immunol. 154, 3194–3203 (1995)
Bustamante, J., Tovar, B.A., Montero, G., Boveris, A.: Early redox changes during rat thymocyte apoptosis. Arch. Biochem. Biophys. 337, 121–128 (1997)
Sun, X., Ross, D.: Quinone-induced apoptosis in human colon adenocarcinoma cells via DT-diaphorase mediated bioactivation. Chem. Biol. Interact. 100, 267–276 (1996)
Albrecht, H., Tschopp, J., Jongeneel, C.V.: Bcl-2 protects from oxidative damage and apoptotic cell death without interfering with activation of NF-kappa B by TNF. FEBS Lett. 351, 45–48 (1994)
Wang, J.H., Redmond, H.P., Watson, R.W., Bouchier-Hayes, D.: Induction of human endothelial cell apoptosis requires both heat shock and oxidative stress responses. Am. J. Physiol. 272, C1543–C1551 (1997)
Atabay, C., Cagnoli, C.M., Kharlamov, E., Ikonomovic, M.D., Nanev, H.: Removal of serum from primary cultures of cerebellar granule neurons induces oxidative stress and DNA fragmentation: protection with antioxidants and glutamate receptor antagonists. J. Neurosci. Res. 43, 465–475 (1996)
Dobmeyer, T.S., Findhammer, S., Dobmeyer, J.M., Klein, S.A., Raffel, B., Hoelzer, D., Helm, E.B., Kabelitz, D., Rossol, R.: Ex vivo induction of apoptosis in lymphocytes is mediated by oxidative stress: role for lymphocyte loss in HIV infection. Free Radical. Biol. Med. 22, 775–785 (1997)
Garcia-Ruiz, C., Colell, A., Mari, M., Morales, A., Fernandez-Checa, J.C.: Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J. Biol. Chem. 272, 11369–11377 (1997)
Johnson, T.M., Yu, Z.X., Ferrans, V.J., Lowenstein, R.A., Finkel, T.: Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 93, 11848–11852 (1996)
Mayer, M., Noble, M.: N-acetyl-L-cysteine is a pluripotent protector against cell death and enhancer of trophic factor-mediated cell survival in vitro. Proc. Natl. Acad. Sci. USA. 91, 7496–7500 (1994)
Iwata, S., Hori, T., Sato, N., Hirota, K., Sasada, T., Mitsui, A., Hirakawa, T., Yodoi, J.: Adult T cell leukemia (ATL)-derived factor/human thioredoxin prevents apoptosis of lymphoid cells induced by L-cystine and glutathione depletion: possible involvement of thiol-mediated redox regulation in apoptosis caused by pro-oxidant state. J. Immunol. 158, 3108–3117 (1997)
Wong, G.H., Elwell, J.H., Oberley, L.W., Goeddel, D.V.: Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell 58, 923–931 (1989)
Chu, Z.L., McKinsey, T.A., Liu, L., Gentry, J.J., Malim, M.H., Ballard, D.W.: Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc. Natl. Acad. Sci. USA 94, 10057–10062 (1997)
Van Antwerp, D.J., Martin, S.J., Kafri, T., Green, D.R., Verma, I.M.: Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science 274, 787–789 (1996)
Li, J.M., Shah, A.M.: Differential NADPH- versus NADH-dependent superoxide production by phagocyte-type endothelial cell NADPH oxidase. Cardiovasc. Res. 52, 477–486 (2001)
Signorelli, P., Hannun, Y.A.: Analysis and quantitation of ceramide. Methods in enzymology 345, 275–294 (2002)
Chatterjee, S., Martin, S.F.: In Membrane lipid signaling in aging and Age-Related Disease, edited by Mark P. Mattson (Lippincott, NY, 2003) pp. 3993–4005.
Carter, W.O., Narayanan, P.K., Robinson, J.P.: Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J. Leukoc. Biol. 55, 253–258 (1994)
Zhang, P., Liu, B., Kang, S.W., Seo, M.S., Rhee, S.G., Obeid, L.M.: Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of Bcl-2. J. Biol. Chem. 272, 30615–30618 (1997)
Sonderfeld, S., Conzelmann, E., Schwarzmann, G., Burg, J., Hinrichs, U., Sandhoff, K.: Incorporation and metabolism of ganglioside GM2 in skin fibroblasts from normal and GM2 gangliosidosis subjects. Eur. J. Biochem. 149, 247–255 (1985)
Kolesnick, R.N., Golde, D.W.: The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 77, 325–328 (1994)
Hannun, Y.A.: The sphingomyelin cycle and the second messenger function of ceramide. J. Biol. Chem. 269, 3125–3128 (1994)
De Maria, R., Lenti, L., Malisan, F., d’Agostino, F., Tomassini, B., Zeuner, A., Rippo, M.R., Testi, R.: Requirement for GD3 ganglioside in CD95- and ceramide-induced apoptosis. Science 277, 1652–1655 (1997)
Bhunia, A.K., Schwarzmann, G., Chatterjee, S.: GD3 recruits reactive oxygen species to induce cell proliferation and apoptosis in human aortic smooth muscle cells. J. Biol. Chem. 277, 16396–16402 (2002)
Zhang, P., Liu, B., Kang, S.W., Seo, M.S., Rhee, S.G., Obeid, L.M.: Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of Bcl-2. J. Biol. Chem. 272, 30615–30618 (1997)
Schutze, S., Machleidt, T., Kronke, M.: The role of diacylglycerol and ceramide in tumor necrosis factor and interleukin-1 signal transduction. J. Leukoc. Biol. 56, 533–541 (1994)
Verheij, M., Bose, R., Lin, X.H., Yao, B., Jarvis, W.D., Grant, S., Birrer, M.J., Szabo, E., Zon, L.I., Kyriakis, J.M., Haimovitz, F.A., Fuks, Z., Kolesnick, R.N.: Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature 380, 75–79 (1996)
Westwick, J.K., Bielawska, A.E., Dbaibo, G., Hannun, Y.A., Brenner, D.A.: Ceramide activates the stress-activated protein kinases. J. Biol. Chem. 270, 22689–22692 (1995)
Radin, N.S., Shayman, J.A., Inokuchi, J.: Metabolic effects of inhibiting glucosylceramide synthesis with PDMP and other substances. Adv. Lipid. Res. 26, 183–213 (1993)
Chatterjee, S., Cleveland, T., Shi, W.Y., Inokuchi, J., Radin, N.S.: Studies of the action of ceramide-like substances (D- and L-PDMP) on sphingolipid glycosyltransferases and purified lactosylceramide synthase. Glycoconj. J. 13, 481–486 (1996)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martin, S.F., Williams, N. & Chatterjee, S. Lactosylceramide is required in apoptosis induced by N-Smase. Glycoconj J 23, 147–157 (2006). https://doi.org/10.1007/s10719-006-7920-8
Issue Date:
DOI: https://doi.org/10.1007/s10719-006-7920-8