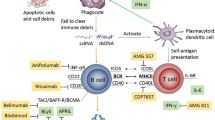
Abstract
Purpose
To investigate the relationships between AMG 811 exposure, concentration changes in serum IFN-γ, and IFN-γ-induced protein 10 (CXCL10), and to identify important contributions of baseline covariates to these relationships.
Methods
A mechanism based pharmacokinetic (PK)-pharmacodynamic (PD) model was developed. A target mediated disposition model was used to describe AMG 811 and target IFN-γ interaction. CXCL10 was predicted to be driven by estimated free IFN-γ levels.
Results
For an average systemic lupus erythematosus (SLE) subject, the linear clearance (CL) of AMG 811 was 0.176 L/day, and the central (Vc) and peripheral (Vp) volumes of distribution were 1.48 and 2.12 L, respectively. Body weight was found to correlate with CL, Vc, Vp, and inter compartment clearance (Q); and age was found to correlate with Vc. The relationship between estimated free serum IFN-γ concentration levels and serum CXCL10 in logarithmic scales was best described by a linear model with slope and intercept estimated to be 0.197 and -0.3, respectively.
Conclusions
The largest observed reduction of serum CXCL10 concentration was achieved at the highest AMG 811 dose tested (180 mg SC). This model enables simulations of AMG 811 PK-PD profiles under various dosing regimens to support future clinical studies.






Similar content being viewed by others
References
Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140:1022–7.
Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–95.
Rauch I, Muller M, Decker T. The regulation of inflammation by interferons and their STATs. JAKSTAT. 2013;2:e23820.
Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR. The role of IFN-gamma in systemic lupus erythematosus: a challenge to the Th1/Th2 paradigm in autoimmunity. Arthritis Res. 2001;3:136–41.
Theofilopoulosand AN, Kono DH. Genetics of systemic autoimmunity and glomerulonephritis in mouse models of lupus. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 16 Suppl 6:65–67 (2001).
Lee JY, Goldman D, Piliero LM, Petri M, Sullivan KE. Interferon-gamma polymorphisms in systemic lupus erythematosus. Genes and immun. 2001;2:254–7.
Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–5.
Funauchi M, Sugishima H, Minoda M, Horiuchi A. Serum level of interferon-gamma in autoimmune diseases. Tohoku J Exp Med. 1991;164:259–67.
Yokoyama H, Takabatake T, Takaeda M, Wada T, Naito T, Ikeda K, et al. Up-regulated MHC-class II expression and gamma-IFN and soluble IL-2R in lupus nephritis. Kidney Int. 1992;42:755–63.
al-Janadi M, al-Balla S, al-Dalaan A, Raziuddin S. Cytokine profile in systemic lupus erythematosus, rheumatoid arthritis, and other rheumatic diseases. J Clin Immunol. 1993;13:58–67.
Narumi S, Takeuchi T, Kobayashi Y, Konishi K. Serum levels of ifn-inducible PROTEIN-10 relating to the activity of systemic lupus erythematosus. Cytokine. 2000;12:1561–5.
Samsonov MY, Tilz GP, Egorova O, Reibnegger G, Balabanova RM, Nassonov EL, et al. Serum soluble markers of immune activation and disease activity in systemic lupus erythematosus. Lupus. 1995;4:29–32.
Hammon M, Herrmann M, Bleiziffer O, Pryymachuk G, Andreoli L, Munoz LE, et al. Role of guanylate binding protein-1 in vascular defects associated with chronic inflammatory diseases. J Cell Mol Med. 2011;15:1582–92.
Bauer JW, Petri M, Batliwalla FM, Koeuth T, Wilson J, Slattery C, et al. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: a validation study. Arthritis Rheum. 2009;60:3098–107.
Martin DA, Boedigheimer M, Amoura Z, Kivitz A, Buyon J, Sanchez-Guerrero J, et al. AMG 811 (anti-IFN-gamma) treatment leads to a reduction in the whole blood IFN-signature and serum CXCL10 in Subjects with Systemic Lupus Erythematosus: Results of two Phase I Studies., the 77th Annual ACR meeting, San Diego, CA 2013.
Hommes DW, Mikhajlova TL, Stoinov S, Stimac D, Vucelic B, Lonovics J, et al. Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn’s disease. Gut. 2006;55:1131–7.
Magerand DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28:507–32.
Gibiansky L, Gibiansky E, Kakkar T, Ma P. Approximations of the target-mediated drug disposition model and identifiability of model parameters. J Pharmacokinet Pharmacodyn. 2008;35:573–91.
Ahn JE, Karlsson MO, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn. 2008;35:401–21.
Mandema JW, Verotta D, Sheiner LB. Building population pharmacokinetic–pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm. 1992;20:511–28.
Doshi S, Chow A, Perez Ruixo JJ. Exposure-response modeling of darbepoetin alfa in anemic patients with chronic kidney disease not receiving dialysis. J Clin Pharmacol. 2010;50:75S–90S.
Radwanski E, Chakraborty A, Van Wart S, Huhn RD, Cutler DL, Affrime MB, et al. Pharmacokinetics and leukocyte responses of recombinant human interleukin-10. Pharm Res. 1998;15:1895–901.
Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14:559–70.
Sutjandra L, Rodriguez RD, Doshi S, Ma M, Peterson MC, Jang GR, et al. Population pharmacokinetic meta-analysis of denosumab in healthy subjects and postmenopausal women with osteopenia or osteoporosis. Clin Pharmacokinet. 2011;50:793–807.
Ng CM, Joshi A, Dedrick RL, Garovoy MR, Bauer RJ. Pharmacokinetic-pharmacodynamic-efficacy analysis of efalizumab in patients with moderate to severe psoriasis. Pharm Res. 2005;22:1088–100.
Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–58.
Manolios N, Schrieber L, Nelson M, Geczy CL. Enhanced interferon-gamma (IFN) production by lymph node cells from autoimmune (MRL/1, MRL/n) mice. Clin Exp Immunol. 1989;76:301–6.
Fanand X, Wuthrich RP. Upregulation of lymphoid and renal interferon-gamma mRNA in autoimmune MRL-Fas (lpr) mice with lupus nephritis. Inflammation. 1997;21:105–12.
Dirksand NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633–59.
Ma P, Yang BB, Wang YM, Peterson M, Narayanan A, Sutjandra L, et al. Population pharmacokinetic analysis of panitumumab in patients with advanced solid tumors. J Clin Pharmacol. 2009;49:1142–56.
Frey N, Grange S, Woodworth T. Population pharmacokinetic analysis of tocilizumab in patients with rheumatoid arthritis. J Clin Pharmacol. 2010;50:754–66.
Tang L, Persky AM, Hochhaus G, Meibohm B. Pharmacokinetic aspects of biotechnology products. J Pharm Sci. 2004;93:2184–204.
Utsal L, Tillmann V, Zilmer M, Maestu J, Purge P, Jurimae J, et al. Elevated serum IL-6, IL-8, MCP-1, CRP, and IFN-gamma levels in 10- to 11-year-old boys with increased BMI. Horm res in paediatr. 2012;78:31–9.
Shurin GV, Yurkovetsky ZR, Chatta GS, Tourkova IL, Shurin MR, Lokshin AE. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine. 2007;39:123–9.
Bergstrandand M, Karlsson MO. Handling data below the limit of quantification in mixed effect models. AAPS J. 2009;11:371–80.
Tokano Y, Morimoto S, Kaneko H, Amano H, Nozawa K, Takasaki Y, et al. Levels of IL-12 in the sera of patients with systemic lupus erythematosus (SLE)–relation to Th1- and Th2-derived cytokines. Clin Exp Immunol. 1999;116:169–73.
Yan X, Mager DE, Krzyzanski W. Selection between Michaelis-Menten and target-mediated drug disposition pharmacokinetic models. J Pharmacokinet Pharmacodyn. 2010;37:25–47.
Ma P. Theoretical considerations of target-mediated drug disposition models: simplifications and approximations. Pharm Res. 2012;29:866–82.
Thompson JA, Lee DJ, Cox WW, Lindgren CG, Collins C, Neraas KA, et al. Recombinant interleukin 2 toxicity, pharmacokinetics, and immunomodulatory effects in a phase I trial. Cancer Res. 1987;47:4202–7.
Gutterman JU, Rosenblum MG, Rios A, Fritsche HA, Quesada JR. Pharmacokinetic study of partially pure gamma-interferon in cancer patients. Cancer Res. 1984;44:4164–71.
Turner PK, Houghton JA, Petak I, Tillman DM, Douglas L, Schwartzberg L, et al. Interferon-gamma pharmacokinetics and pharmacodynamics in patients with colorectal cancer. Cancer Chemother Pharmacol. 2004;53:253–60.
Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–6.
Kong KO, Tan AW, Thong BY, Lian TY, Cheng YK, Teh CL, et al. Enhanced expression of interferon-inducible protein-10 correlates with disease activity and clinical manifestations in systemic lupus erythematosus. Clin Exp Immunol. 2009;156:134–40.
Rose T, Grutzkau A, Hirseland H, Huscher D, Dahnrich C, Dzionek A, et al. IFNalpha and its response proteins, IP-10 and SIGLEC-1, are biomarkers of disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2013;72:1639–45.
Dominguez-Gutierrez PR, Ceribelli A, Satoh M, Sobel ES, Reeves WH, Chan EK. Reduced levels of CCL2 and CXCL10 in systemic lupus erythematosus patients under treatment with prednisone, mycophenolate mofetil, or hydroxychloroquine, except in a high STAT1 subset. Arthritis res & ther. 2014;16:R23.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors thank all the patients, the investigators, and their medical, nursing, and laboratory staff who participated in the clinical studies included in the present analysis. These studies were sponsored by Amgen Inc., which was involved in the study design, data collection, analysis, interpretation, writing the manuscript, and the decision to submit the manuscript for publication. Ping Chen, Thuy Vu, Adimoolam Narayanan, Winnie Sohn, Jin Wang, Michael Boedigheimer, Andrew Welcher, Barbara Sullivan, David Martin, and Juan Jose Perez Ruixo are employees of Amgen Inc. and own stock in Amgen Inc. at the time this analysis was conducted. Peiming Ma is a former employee of Amgen Inc. The authors have no other conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, P., Vu, T., Narayanan, A. et al. Pharmacokinetic and Pharmacodynamic Relationship of AMG 811, An Anti-IFN-γ IgG1 Monoclonal Antibody, in Patients with Systemic Lupus Erythematosus. Pharm Res 32, 640–653 (2015). https://doi.org/10.1007/s11095-014-1492-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1492-2