Abstract
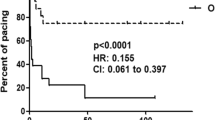
One of the strongest clinical associations with autoantibodies against components of the SSA/Ro–SSB/La ribonucleoprotein complex is the development of congenital heart block in an offspring, an alarming prospect facing 2% of primigravid mothers with these reactivities. This risk is increased tenfold in women who have had a previous child with congenital heart block. Accumulated evidence suggests that anti-SSA/Ro and anti-SSB/La antibodies are necessary but insufficient for fetal disease. Basic and clinical research is heavily focused on identifying fetal and environmental factors that convert disease susceptibility to disease development. A disturbing observation that has emerged from current research efforts is the rapidity of disease progression, with advanced heart block and life-threatening cardiomyopathy being observed less than 2 weeks after detection of a normal sinus rhythm. Once third-degree block is unequivocally identified, reversal has never been achieved, despite dexamethasone treatment. Accordingly, strategies aimed at preventing disease before irrevocable scarring ensues assume a high priority. One approach has been the implementation of serial echocardiography to monitor for a prolonged PR interval. Intravenous immunoglobulin is being evaluated as a potential prophylactic approach in mothers who have previously had an affected child.
Key Points
-
Mothers with anti-SSA/Ro antibodies face a 2% risk of having a child with congenital heart block if it is a first pregnancy or if previous babies have all been healthy
-
A previous child with congenital heart block raises the risk of having another by almost tenfold
-
Normal sinus rhythm can progress to complete block in 7 days; thus, frequent monitoring of a pregnancy in a mother with anti-SSA/Ro antibodies is appropriate
-
A mechanical PR interval of greater than 150 ms is consistent with first-degree block, and warrants an immediate discussion regarding the use of a fluorinated steroid to potentially reverse the situation
-
Intravenous immunoglobulin is currently being evaluated as a prophylactic therapy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Buyon JP et al. (1996) The effects of pregnancy on autoimmune diseases. Clin Immunol Immunopath 78: 99–104
Buyon JP and Clancy RM (2006) Neonatal lupus. In Dubois' Lupus Erythematosus, edn 7, 1058–1080 (Eds Wallace DJ and Hahn BH) Philadelphia: Lippincott Williams & Wilkins
Leach JL et al. (1996) Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol 157: 3317–3322
Jaeggi ET et al. (2002) Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. J Am Coll Cardiol 39: 130–137
Moak JP et al. (2001) Congenital heart block: development of late-onset cardiomyopathy, a previously underappreciated sequela. J Am Coll Cardiol 37: 238–242
Buyon JP et al. (1998) Autoimmune-associated congenital heart block: mortality, morbidity, and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol 31: 1658–1666
Waltuck J and Buyon J (1994) Autoantibody-associated congenital heart block: outcome in mothers and children. Annals Int Med 120: 544–551
Brucato A et al. (2001) Risk of congenital heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis. Arthritis Rheum 44: 1832–1835
Friedman DM et al. (2008) Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation 117: 485–493
Salomonsson S et al. (2002) A serologic marker for fetal risk of congenital heart block. Arthritis Rheum 46: 1233–1241
Salomonsson S et al. (2005) Ro/SSA autoantibodies directly bind cardiomyocytes, disturb calcium homeostasis, and mediate congenital heart block. J Exp Med 201: 11–17
Buyon JP et al. (1994) Autoantibody responses to the “native” 52 kDa SS-A/Ro protein in neonatal lupus syndromes, systemic lupus erythematosus and Sjögren's syndrome. J Immunology 152: 75–84
Clancy RM et al. (2005) Maternal antibody responses to the 52 kDa SSA/Ro p200 peptide and the development of fetal conduction defects. Arthritis Rheum 52: 3079–3086
Solomon DG et al. (2003) Birth order, gender and recurrence rate in autoantibody-associated congenital heart block: implications for pathogenesis and family counseling. Lupus 12: 646–647
Gordon P et al. (2004) Anti-52 kDa Ro, anti-60 kDa Ro, and anti-La antibody profiles in neonatal lupus. J Rheum 31: 2480–2487
Vincent A et al. (2000) Molecular targets for autoimmune and genetic disorders of neuromuscular transmission. Eur J Biochem 267: 6717–6728
Kreier JP (2002) Infection, Resistance and Immunity, edn 2. Oxford, UK: Taylor & Francis
Alexander E et al. (1992) Anti-Ro/SS-A antibodies in the pathophysiology of congenital heart block in neonatal lupus syndrome, an experimental model: in vitro electrophysiologic and immunocytochemical studies. Arthritis Rheum 35: 176–189
Garcia S et al. (1994) Cellular mechanism of the conduction abnormalities induced by serum from anti-Ro/SSA-positive patients in rabbit hearts. J Clin Invest 93: 718–724
Boutjdir M et al. (1998) Serum and IgG from the mother of a child with congenital heart block induce conduction abnormalities and inhibit L-type calcium channels in a rat heart model. Pediatr Res 80: 354–362
Buyon JP et al. (2002) Cardiac 5-HT4 serotoninergic receptors, 52 kD SSA/Ro and autoimmune-associated congenital heart block. J Autoimmunity 19: 79–86
Kamel R et al. (2005) Autoantibodies against the serotoninergic 5-HT4 receptor and congenital heart block: a reassessment. J Autoimmun 25: 72–76
Clancy RM et al. (2004) Immunohistologic evidence supports apoptosis, IgG deposition and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum 50: 173–182
Clancy RM et al. (2006) Impaired clearance of apoptotic cardiocytes linked to anti-SSA/Ro-SSB/La antibodies in pathogenesis of congenital heart block. J Clin Investigation 116: 2413–2422
Clancy RM et al. (2002) Transdifferentiation of cardiac fibroblasts, a fetal factor in anti-SSA/Ro-SSB/La antibody-mediated congenital heart block. J Immunol 169: 2156–2163
Miranda-Carús ME et al. (2000) Anti-SSA/Ro and anti-SSB/La autoantibodies bind the surface of apoptotic fetal cardiocytes and promote secretion of tumor necrosis factor α by macrophages. J Immunol 165: 5345–5351
Clancy RM et al. (2003) Cytokine polymorphisms and histologic expression in autopsy studies: contribution of TNF-α and TGFβ1 to the pathogenesis of autoimmune-associated congenital heart block. J Immunol 171: 3253–3261
Clancy RM et al. (2007) Role of hypoxia and cAMP in the transdifferentiation of human fetal cardiac fibroblasts: implications for progression to scarring in autoimmune-associated congenital heart block. Arthritis Rheum 56: 4120–4131
McCue CM et al. (1977) Congenital heart block in newborns of mothers with connective tissue disease. Circulation 56: 82–90
Geggel RL et al. (1988) Postnatal progression from second- to third-degree heart block in neonatal lupus syndrome. J Ped 113: 1049–1052
Askanase AD et al. (2002) Spectrum and progression of conduction abnormalities in infants born to mothers with anti-Ro/La antibodies. Lupus 11: 145–151
Saleeb S et al. (1999) Comparison of treatment with fluorinated glucocorticoids to the natural history of autoantibody-associated congenital heart block. Arthritis Rheum 42: 2335–2345
Jaeggi ET et al. (2004) Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation 110: 1542–1548
Rosenthal D et al. (1998) A new therapeutic approach to the fetus with congenital complete heart block: pre-emptive, targeted therapy with dexamethasone. Obstet Gynecol 92: 689–691
Breur JMPJ et al. (2004) Treatment of fetal heart block with maternal steroid therapy: case report and review of the literature. Ultrasound Obstet Gynecol 24: 467–472
Brucato A et al. (2006) Normal neuropsychological development in children with congenital complete heart block who may or may not be exposed to high-dose dexamethasone in utero. Ann Rheum Dis 65: 1422–1426
Airo' P et al. (2006) Characterization of T-cell population in children with prolonged fetal exposure to dexamethasone for anti-Ro/SS-A antibodies associated congenital heart block. Lupus 15: 553–561
Sonesson SE et al. (2004) Signs of first-degree heart block occur in one-third of fetuses of pregnant women with anti-SSA/Ro 52 kD antibodies. Arthritis Rheum 50: 1253–1261
Andelfinger G et al. (2001) Reference values for time intervals between atrial and ventricular contractions of the fetal heart measured by two Doppler techniques. Am J Cardiol 88: 1433–1436
Clancy RM et al. (2002) Transdifferentiation of cardiac fibroblasts, a fetal factor in anti-SSA/Ro-SSB/La antibody-mediated congenital heart block. J Immunol 169: 2156–2163
Kaaja R and Julkunen H (2003) Prevention of recurrence of congenital heart block with intravenous immunoglobulin and corticosteroid therapy: comment on the editorial by Buyon et al. [letter]. Arthritis Rheum 48: 281–282
Looney RJ and Huggins J (2006) Use of intravenous immunoglobulin (IVIG). Best Pract Res Clin Haematol 18: 3–25
Samuelsson A et al. (2001) Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291: 445–446
Nimmerjahn F and Ravetch JV (2008) Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol 26: 513–533
Anthony RM et al. (2008) Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 320: 373–376
Preventive IVIG Therapy for Congenital Heart Block (PITCH) [http://clinicaltrials.gov/ct2/show/NCT00460928?term=NCT00460928&rank=1] (accessed 13 January 2009)
Acknowledgements
This work was funded by an NIH-NIAMS grant (Maternal Autoantibodies: Pathogenesis of Neonatal Lupus), an NIH contract (Research Registry for Neonatal Lupus) and an NIH-NIAMS grant (the PRIDE study) to Dr Buyon, and an American Heart Association, Heritage Affiliate grant to Dr Clancy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Buyon, J., Clancy, R. & Friedman, D. Cardiac manifestations of neonatal lupus erythematosus: guidelines to management, integrating clues from the bench and bedside. Nat Rev Rheumatol 5, 139–148 (2009). https://doi.org/10.1038/ncprheum1018
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum1018
This article is cited by
-
Causes of fetal third-degree atrioventricular block and use of hydroxychloroquine in pregnant women with Ro/La antibodies
Clinical Rheumatology (2019)
-
Neonatal lupus erythematosus: a cutaneous cases based update
Italian Journal of Pediatrics (2016)
-
Management of Lupus Nephritis
Current Treatment Options in Rheumatology (2016)
-
Managing pregnancy in inflammatory rheumatological diseases
Arthritis Research & Therapy (2011)