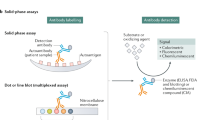
Abstract
Systemic lupus erythematosus (SLE) is a prototypic autoimmune disease characterized by antinuclear antibodies (ANAs) that form immune complexes that mediate pathogenesis by tissue deposition or cytokine induction. Some ANAs bind DNA or associated nucleosome proteins, whereas other ANAs bind protein components of complexes of RNA and RNA-binding proteins (RBPs). Levels of anti-DNA antibodies can fluctuate widely, unlike those of anti-RBP antibodies, which tend to be stable. Because anti-DNA antibody levels can reflect disease activity, repeat testing is common; by contrast, a single anti-RBP antibody determination is thought to suffice for clinical purposes. Experience from clinical trials of novel therapies has provided a new perspective on ANA expression during disease, as many patients with SLE are ANA negative at screening despite previously testing positive. Because trial results suggest that patients who are ANA negative might not respond to certain agents, screening strategies now involve ANA and anti-DNA antibody testing to identify patients with so-called ‘active, autoantibody-positive SLE’. Evidence suggests that ANA responses can decrease over time because of the natural history of disease or the effects of therapy. Together, these findings suggest that, during established disease, more regular serological testing could illuminate changes relevant to pathogenesis and disease status.
Key points
-
Antinuclear antibodies (ANAs) bind DNA, RNA and complexes of nucleic acids and protein.
-
In addition to ANAs, anti-DNA and anti-Sm antibodies are part of the classification criteria for systemic lupus erythematosus (SLE).
-
Anti-DNA antibodies and antibodies that recognize RNA-binding proteins show distinct patterns of expression that relate to their origin from different B cell populations.
-
ANAs can mediate events in the pathogenesis of SLE as either free antibodies or as immune complexes.
-
Amounts of ANAs in patients with SLE can change over time as a result of the natural history of the disease or the effects of immunosuppressive agents.
-
Rescreening of ANA levels after disease onset could provide important information about disease mechanisms and disease status.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kaul, A. et al. Systemic lupus erythematosus. Nat. Rev. Dis. Prim. 2, 16039 (2016).
Tsokos, G. C., Lo, M. S., Costa Reis, P. & Sullivan, K. E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 716–730 (2016).
Agmon-Levin, N. et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann. Rheum. Dis. 73, 17–23 (2014).
Pisetsky, D. S. Antinuclear antibody testing: misunderstood or misbegotten? Nat. Rev. Rheumatol. 13, 495–502 (2017).
Damoiseaux, J. et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: the International Consensus on ANA patterns (ICAP) perspective. Ann. Rheum. Dis. 78, 879–889 (2019).
Wallace, D. J. et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. 61, 1168–1178 (2009).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 63, 3918–3930 (2011).
Pisetsky, D. S., Rovin, B. H. & Lipsky, P. E. New perspectives in rheumatology: biomarkers as entry criteria for clinical trials of new therapies for systemic lupus erythematosus: the example of antinuclear antibodies and anti-DNA. Arthritis Rheumatol. 69, 487–493 (2017).
Hueber, W., Utz, P. J., Steinman, L. & Robinson, W. H. Autoantibody profiling for the study and treatment of autoimmune disease. Arthritis Res. 4, 290–295 (2002).
Li, Q. Z. et al. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin. Exp. Immunol. 147, 60–70 (2007).
Deng, X. et al. Utility of antinuclear antibody screening by various methods in a clinical laboratory patient cohort. J. Appl. Lab. Med. 1, 36–46 (2016).
Olsen, N. J., Choi, M. Y. & Fritzler, M. J. Emerging technologies in autoantibody testing for rheumatic diseases. Arthritis Res. Ther. 19, 172 (2017).
Pisetsky, D. S., Bossuyt, X. & Meroni, P. L. ANA as an entry criterion for the classification of SLE. Autoimmun. Rev. 18, 102400 (2019).
Choi, M. Y. et al. Antinuclear antibody-negative systemic lupus erythematosus in an international inception cohort. Arthritis Care Res. 71, 893–902 (2019).
Leuchten, N. et al. Performance of antinuclear antibodies for classifying systemic lupus erythematosus: a systematic literature review and meta-regression of diagnostic data. Arthritis Care Res. 70, 428–438 (2018).
Aringer, M. et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 71, 1400–1412 (2019).
Aringer, M. et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheum. Dis. 78, 1151–1159 (2019).
Tan, E. M. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25, 1271–1277 (1982).
Petri, M. et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686 (2012).
Chevrier, M., Jordan, J., Schreiter, J. & Benson, J. Comparitive analysis of anti-nuclear antibody testing using blinded replicate samples reveals variability between commercial testing laboratories [abstract]. Arthritis Rheumatol. 68, 2809 (2016).
Pisetsky, D. S., Spencer, D. M., Lipsky, P. E. & Rovin, B. H. Assay variation in the detection of antinuclear antibodies in the sera of patients with established SLE. Ann. Rheum. Dis. 77, 911–913 (2018).
Binder, S. R., Genovese, M. C., Merrill, J. T., Morris, R. I. & Metzger, A. L. Computer-assisted pattern recognition of autoantibody results. Clin. Diagn. Lab. Immunol. 12, 1353–1357 (2005).
Park, Y. et al. Automated versus conventional microscopic interpretation of antinuclear antibody indirect immunofluorescence test. Ann. Clin. Lab. Sci. 49, 127–133 (2019).
Pisetsky, D. S. Anti-DNA antibodies — quintessential biomarkers of SLE. Nat. Rev. Rheumatol. 12, 102–110 (2016).
Viana, V. T., Durcan, L., Bonfa, E. & Elkon, K. B. Ribosomal P antibody: 30 years on the road. Lupus 26, 453–462 (2017).
Choi, M. Y., FitzPatrick, R. D., Buhler, K., Mahler, M. & Fritzler, M. J. A review and meta-analysis of anti-ribosomal P autoantibodies in systemic lupus erythematosus. Autoimmun. Rev. 19, 102463 (2020).
Ghiggeri, G. M. et al. An update on antibodies to necleosome components as biomarkers of sistemic lupus erythematosus and of lupus flares. Int. J. Mol. Sci. 20, 5799 (2019).
Mehra, S. & Fritzler, M. J. The spectrum of anti-chromatin/nucleosome autoantibodies: independent and interdependent biomarkers of disease. J. Immunol. Res. 2014, 368274 (2014).
Rekvig, O. P., van der Vlag, J. & Seredkina, N. Review: antinucleosome antibodies: a critical reflection on their specificities and diagnostic impact. Arthritis Rheumatol. 66, 1061–1069 (2014).
Rekvig, O. P. The anti-DNA antibody: origin and impact, dogmas and controversies. Nat. Rev. Rheumatol. 11, 530–540 (2015).
Stollar, B. D. Antibodies to DNA. Crit. Rev. Biochem. 20, 1–36 (1986).
Jang, Y. J. & Stollar, B. D. Anti-DNA antibodies: aspects of structure and pathogenicity. Cell. Mol. Life Sci. 60, 309–320 (2003).
Schur, P. H. & Sandson, J. Immunologic factors and clinical activity in systemic lupus erythematosus. N. Engl. J. Med. 278, 533–538 (1968).
McCarty, G. A., Rice, J. R., Bembe, M. L. & Pisetsky, D. S. Independent expression of autoantibodies in systemic lupus erythematosus. J. Rheumatol. 9, 691–695 (1982).
Ward, M. M., Pisetsky, D. S. & Christenson, V. D. Antidouble stranded DNA antibody assays in systemic lupus erythematosus: correlations of longitudinal antibody measurements. J. Rheumatol. 16, 609–613 (1989).
ter Borg, E. J., Horst, G., Hummel, E. J., Limburg, P. C. & Kallenberg, C. G. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 33, 634–643 (1990).
Portanova, J. P., Arndt, R. E., Tan, E. M. & Kotzin, B. L. Anti-histone antibodies in idiopathic and drug-induced lupus recognize distinct intrahistone regions. J. Immunol. 138, 446–451 (1987).
Vaglio, A. et al. Drug-induced lupus: traditional and new concepts. Autoimmun. Rev. 17, 912–918 (2018).
Migliorini, P., Baldini, C., Rocchi, V. & Bombardieri, S. Anti-Sm and anti-RNP antibodies. Autoimmunity 38, 47–54 (2005).
Schulte-Pelkum, J., Fritzler, M. & Mahler, M. Latest update on the Ro/SS-A autoantibody system. Autoimmun. Rev. 8, 632–637 (2009).
To, C. H. & Petri, M. Is antibody clustering predictive of clinical subsets and damage in systemic lupus erythematosus? Arthritis Rheum. 52, 4003–4010 (2005).
Ching, K. H. et al. Two major autoantibody clusters in systemic lupus erythematosus. PLoS One 7, e32001 (2012).
St Clair, E. W. et al. Expression of autoantibodies to recombinant (U1) RNP-associated 70K antigen in systemic lupus erythematosus. Clin. Immunol. Immunopathol. 54, 266–280 (1990).
Sharp, G. The origin of mixed connective tissue disease: a stimulus for autoimmune disease research. Lupus 18, 1031–1032 (2009).
Arbuckle, M. R. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 349, 1526–1533 (2003).
Heinlen, L. D. et al. 60 kD Ro and nRNP A frequently initiate human lupus autoimmunity. PLoS One 5, e9599 (2010).
Pisetsky, D. S., McCarty, G. A. & Peters, D. V. Mechanisms of autoantibody production in autoimmune MRL mice. J. Exp. Med. 152, 1302–1310 (1980).
Eisenberg, R. A., Craven, S. Y., Warren, R. W. & Cohen, P. L. Stochastic control of anti-Sm autoantibodies in MRL/Mp-lpr/lpr mice. J. Clin. Invest. 80, 691–697 (1987).
Catalina, M. D. et al. Patient ancestry significantly contributes to molecular heterogeneity of systemic lupus erythematosus. JCI Insight 110, 102359 (2020).
Tikly, M., Burgin, S., Mohanlal, P., Bellingan, A. & George, J. Autoantibodies in black South Africans with systemic lupus erythematosus: spectrum and clinical associations. Clin. Rheumatol. 15, 143–147 (1996).
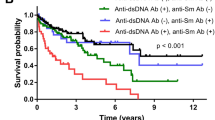
Alba, P. et al. Anti-dsDNA, anti-Sm antibodies, and the lupus anticoagulant: significant factors associated with lupus nephritis. Ann. Rheum. Dis. 62, 556–560 (2003).
Ko, K., Koldobskaya, Y., Rosenzweig, E. & Niewold, T. B. Activation of the interferon pathway is dependent upon autoantibodies in African-American SLE patients, but not in European-American SLE patients. Front. Immunol. 4, 309 (2013).
Elbagir, S. et al. Sudanese and Swedish patients with systemic lupus erythematosus: immunological and clinical comparisons. Rheumatology 59, 968–978 (2020).
Riley, R. L., Addis, D. J. & Taylor, R. P. Stability of DNA/anti-DNA complexes. II. Salt lability and avidity. J. Immunol. 124, 1–7 (1980).
Smeenk, R., van der Lelij, G. & Aarden, L. Avidity of antibodies to dsDNA: comparison of IFT on Crithidia luciliae, Farr assay, and PEG assay. J. Immunol. 128, 73–78 (1982).
Smeenk, R. J., Van Rooijen, A. & Swaak, T. J. Dissociation studies of DNA/anti-DNA complexes in relation to anti-DNA avidity. J. Immunol. Methods 109, 27–35 (1988).
Haugbro, K., Nossent, J. C., Winkler, T., Figenschau, Y. & Rekvig, O. P. Anti-dsDNA antibodies and disease classification in antinuclear antibody positive patients: the role of analytical diversity. Ann. Rheum. Dis. 63, 386–394 (2004).
Compagno, M. et al. Clinical phenotype associations with various types of anti-dsDNA antibodies in patients with recent onset of rheumatic symptoms. Results from a multicentre observational study. Lupus Sci. Med. 1, e000007 (2014).
Pisetsky, D. S. Antinuclear antibodies in rheumatic disease: a proposal for a function-based classification. Scand. J. Immunol. 76, 223–228 (2012).
Mahajan, A., Herrmann, M. & Munoz, L. E. Clearance deficiency and cell death pathways: a model for the pathogenesis of SLE. Front. Immunol. 7, 35 (2016).
Elkon, K. B. Review: cell death, nucleic acids, and immunity: inflammation beyond the grave. Arthritis Rheumatol. 70, 805–816 (2018).
Al-Mayouf, S. M. et al. Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat. Genet. 43, 1186–1188 (2011).
Sisirak, V. et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell 166, 88–101 (2016).
Soni, C. & Reizis, B. DNA as a self-antigen: nature and regulation. Curr. Opin. Immunol. 55, 31–37 (2018).
Weisenburger, T. et al. Epistatic interactions between mutations of deoxyribonuclease 1-like 3 and the inhibitory Fc gamma receptor IIB result in very early and massive autoantibodies against double-stranded DNA. Front. Immunol. 9, 1551 (2018).
Crow, Y. J. & Manel, N. Aicardi-Goutieres syndrome and the type I interferonopathies. Nat. Rev. Immunol. 15, 429–440 (2015).
Uggenti, C., Lepelley, A. & Crow, Y. J. Self-awareness: nucleic acid-driven inflammation and the type I interferonopathies. Annu. Rev. Immunol. 37, 247–267 (2019).
Nielsen, C. T. et al. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum. 64, 1227–1236 (2012).
Zirngibl, M. et al. Loading of nuclear autoantigens prototypically recognized by systemic lupus erythematosus sera into late apoptotic vesicles requires intact microtubules and myosin light chain kinase activity. Clin. Exp. Immunol. 179, 39–49 (2015).
Mobarrez, F. et al. Microparticles in the blood of patients with SLE: size, content of mitochondria and role in circulating immune complexes. J. Autoimmun. 102, 142–149 (2019).
Abrass, C. K., Nies, K. M., Louie, J. S., Border, W. A. & Glassock, R. J. Correlation and predictive accuracy of circulating immune complexes with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 23, 273–282 (1980).
Lock, R. J. & Unsworth, D. J. Measurement of immune complexes is not useful in routine clinical practice. Ann. Clin. Biochem. 37, 253–261 (2000).
Wener, M. H. Tests for circulating immune complexes. Methods Mol. Biol. 1134, 47–57 (2014).
Putterman, C. et al. Cell-bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard complement measurements. Lupus Sci. Med. 1, e000056 (2014).
Trouw, L. A., Pickering, M. C. & Blom, A. M. The complement system as a potential therapeutic target in rheumatic disease. Nat. Rev. Rheumatol. 13, 538–547 (2017).
Nowling, T. K. & Gilkeson, G. S. Mechanisms of tissue injury in lupus nephritis. Arthritis Res. Ther. 13, 250 (2011).
Ho, A., Magder, L. S., Barr, S. G. & Petri, M. Decreases in anti-double-stranded DNA levels are associated with concurrent flares in patients with systemic lupus erythematosus. Arthritis Rheum. 44, 2342–2349 (2001).
Zykova, S. N., Tveita, A. A. & Rekvig, O. P. Renal Dnase1 enzyme activity and protein expression is selectively shut down in murine and human membranoproliferative lupus nephritis. PLoS One 5, 0012096 (2010).
Pedersen, H. L. et al. Lupus nephritis: low urinary DNase I levels reflect loss of renal DNase I and may be utilized as a biomarker of disease progression. J. Pathol. Clin. Res. 4, 193–203 (2018).
Vallin, H., Perers, A., Alm, G. V. & Ronnblom, L. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J. Immunol. 163, 6306–6313 (1999).
Shrivastav, M. & Niewold, T. B. Nucleic acid sensors and type I interferon production in systemic lupus erythematosus. Front. Immunol. 4, 319 (2013).
Sharma, S., Fitzgerald, K. A., Cancro, M. P. & Marshak-Rothstein, A. Nucleic acid-sensing receptors: rheostats of autoimmunity and autoinflammation. J. Immunol. 195, 3507–3512 (2015).
Barrat, F. J., Elkon, K. B. & Fitzgerald, K. A. Importance of nucleic acid recognition in inflammation and autoimmunity. Annu. Rev. Med. 67, 323–336 (2016).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Li, T. & Chen, Z. J. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 215, 1287–1299 (2018).
Lovgren, T., Eloranta, M. L., Bave, U., Alm, G. V. & Ronnblom, L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 50, 1861–1872 (2004).
Kirou, K. A. et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 52, 1491–1503 (2005).
Weckerle, C. E. et al. Network analysis of associations between serum interferon-alpha activity, autoantibodies, and clinical features in systemic lupus erythematosus. Arthritis Rheum. 63, 1044–1053 (2011).
Jackson, S. W. et al. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J. Immunol. 192, 4525–4532 (2014).
Christensen, S. R. et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417–428 (2006).
Tilstra, J. S. et al. B cell-intrinsic TLR9 expression is protective in murine lupus. J. Clin. Invest. 130, 3172–3187 (2020).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl Med. 3, 73ra20 (2011).
van Dam, L. S. et al. Intrinsically distinct role of neutrophil extracellular trap formation in antineutrophil cytoplasmic antibody-associated vasculitis compared to systemic lupus erythematosus. Arthritis Rheumatol. 71, 2047–2058 (2019).
Gupta, S. & Kaplan, M. J. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat. Rev. Nephrol. 12, 402–413 (2016).
Apel, F., Zychlinsky, A. & Kenny, E. F. The role of neutrophil extracellular traps in rheumatic diseases. Nat. Rev. Rheumatol. 14, 467–475 (2018).
Kowal, C. et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl Acad. Sci. USA 103, 19854–19859 (2006).
Nestor, J. et al. Lupus antibodies induce behavioral changes mediated by microglia and blocked by ACE inhibitors. J. Exp. Med. 215, 2554–2566 (2018).
Azzouz, D. et al. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann. Rheum. Dis. 78, 947–956 (2019).
Silverman, G. J., Azzouz, D. F. & Alekseyenko, A. V. Systemic lupus erythematosus and dysbiosis in the microbiome: cause or effect or both? Curr. Opin. Immunol. 61, 80–85 (2019).
Diamond, B. & Lipsky, P. E. in Harrison’s Principles of Internal Medicine 20th edn, Ch. 348 (eds Jameson, J. L., Fauci, A. S., Kasper, D. L., Hauser, S. L., Longo, D. L. & Loscalzo, J.) 2510–2515 (McGraw-Hill Education 2018).
Bombardier, C., Gladman, D. D., Urowitz, M. B., Caron, D. & Chang, C. H. Derivation of the SLEDAI. A disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis Rheum. 35, 630–640 (1992).
Gladman, D. D., Ibanez, D. & Urowitz, M. B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 29, 288–291 (2002).
St Clair, E. W., Burch, J. A. Jr, Ward, M. M., Keene, J. D. & Pisetsky, D. S. Temporal correlation of antibody responses to different epitopes of the human La autoantigen. J. Clin. Invest. 85, 515–521 (1990).
Ferraccioli, G. & Houssiau, F. A. Which B-cell subset should we target in lupus? Ann. Rheum. Dis. 72, 1891–1892 (2013).
Tipton, C. M. et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat. Immunol. 16, 755–765 (2015).
Hiepe, F. & Radbruch, A. Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat. Rev. Nephrol. 12, 232–240 (2016).
Tipton, C. M., Hom, J. R., Fucile, C. F., Rosenberg, A. F. & Sanz, I. Understanding B-cell activation and autoantibody repertoire selection in systemic lupus erythematosus: a B-cell immunomics approach. Immunol. Rev. 284, 120–131 (2018).
Stohl, W. et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 64, 2328–2337 (2012).
Amanna, I. J., Carlson, N. E. & Slifka, M. K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357, 1903–1915 (2007).
Mannik, M., Merrill, C. E., Stamps, L. D. & Wener, M. H. Multiple autoantibodies form the glomerular immune deposits in patients with systemic lupus erythematosus. J. Rheumatol. 30, 1495–1504 (2003).
Vilá, L. M. et al. Clinical and prognostic value of autoantibodies in Puerto Ricans with systemic lupus erythematosus. Lupus 15, 892–898 (2006).
Ahlin, E. et al. Autoantibodies associated with RNA are more enriched than anti-dsDNA antibodies in circulating immune complexes in SLE. Lupus 21, 586–595 (2012).
Arroyo-Avila, M. et al. Clinical associations of anti-Smith antibodies in PROFILE: a multi-ethnic lupus cohort. Clin. Rheumatol. 34, 1217–1223 (2015).
Maddison, P. J. & Reichlin, M. Deposition of antibodies to a soluble cytoplasmic antigen in the kidneys of patients with systemic lupus erythematosus. Arthritis Rheum. 22, 858–863 (1979).
Venables, P. J., Yi, T., Woodrow, D. F., Moss, J. & Maini, R. N. Relationship of precipitating antibodies to soluble cellular antigens and histologically defined renal lesions in systemic lupus erythematosus. Ann. Rheum. Dis. 42, 17–22 (1983).
Simmons-O’Brien, E. et al. One hundred anti-Ro (SS-A) antibody positive patients: a 10-year follow-up. Medicine 74, 109–130 (1995).
Barada, F. A. Jr, Andrews, B. S., Davis, J. S. IV & Taylor, R. P. Antibodies to Sm in patients with systemic lupus erythematosus. Correlation of Sm antibody titers with disease activity and other laboratory parameters. Arthritis Rheum. 24, 1236–1244 (1981).
Praprotnik, S., Bozic, B., Kveder, T. & Rozman, B. Fluctuation of anti-Ro/SS-A antibody levels in patients with systemic lupus erythematosus and Sjögren’s syndrome: a prospective study. Clin. Exp. Rheumatol. 17, 63–68 (1999).
Tench, C. M. & Isenberg, D. A. The variation in anti-ENA characteristics between different ethnic populations with systemic lupus erythematosus over a 10-year period. Lupus 9, 374–376 (2000).
Faria, A. C., Barcellos, K. S. & Andrade, L. E. Longitudinal fluctuation of antibodies to extractable nuclear antigens in systemic lupus erythematosus. J. Rheumatol. 32, 1267–1272 (2005).
Ippolito, A. et al. Autoantibodies in systemic lupus erythematosus: comparison of historical and current assessment of seropositivity. Lupus 20, 250–255 (2011).
Fisher, D. E., Reeves, W. H., Wisniewolski, R., Lahita, R. G. & Chiorazzi, N. Temporal shifts from Sm to ribonucleoprotein reactivity in systemic lupus erythematosus. Arthritis Rheum. 28, 1348–1355 (1985).
Butler, W. T. & Rossen, R. D. Effects of corticosteroids on immunity in man. I. Decreased serum IgG concentration caused by 3 or 5 days of high doses of methylprednisolone. J. Clin. Invest. 52, 2629–2640 (1973).
Bijl, M., Horst, G., Bootsma, H., Limburg, P. C. & Kallenberg, C. G. Mycophenolate mofetil prevents a clinical relapse in patients with systemic lupus erythematosus at risk. Ann. Rheum. Dis. 62, 534–539 (2003).
Ng, K. P. et al. B cell depletion therapy in systemic lupus erythematosus: long-term follow-up and predictors of response. Ann. Rheum. Dis. 66, 1259–1262 (2007).
Tew, G. W. et al. Baseline autoantibody profiles predict normalization of complement and anti-dsDNA autoantibody levels following rituximab treatment in systemic lupus erythematosus. Lupus 19, 146–157 (2010).
Eickenberg, S. et al. Mycophenolic acid counteracts B cell proliferation and plasmablast formation in patients with systemic lupus erythematosus. Arthritis Res. Ther. 14, R110 (2012).
Lewis, M. J. et al. Autoantibodies targeting TLR and SMAD pathways define new subgroups in systemic lupus erythematosus. J. Autoimmun. 91, 1–12 (2018).
Pisetsky, D. S., Thompson, D. K., Wajdula, J., Diehl, A. & Sridharan, S. Variability in antinuclear antibody testing to assess patient eligibility for clinical trials of novel treatments for systemic lupus erythematosus. Arthritis Rheumatol. 71, 1534–1538 (2019).
Putterman, C. et al. The SLE-key test serological signature: new insights into the course of lupus. Rheumatology 57, 1632–1640 (2018).
An, J., Minie, M., Sasaki, T., Woodward, J. J. & Elkon, K. B. Antimalarial drugs as immune modulators: new mechanisms for old drugs. Annu. Rev. Med. 68, 317–330 (2017).
Yurasov, S. et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201, 703–711 (2005).
Yurasov, S. et al. Persistent expression of autoantibodies in SLE patients in remission. J. Exp. Med. 203, 2255–2261 (2006).
Dorner, T. & Lipsky, P. E. B cells: depletion or functional modulation in rheumatic diseases. Curr. Opin. Rheumatol. 26, 228–236 (2014).
Suurmond, J. et al. Patterns of ANA+B cells for SLE patient stratification. JCI Insight 4, e127885 (2019).
Suurmond, J. et al. Loss of an IgG plasma cell checkpoint in patients with lupus. J. Allergy. Clin. Immunol. 143, 1586–1597 (2019).
McMichael, A. J., Borrow, P., Tomaras, G. D., Goonetilleke, N. & Haynes, B. F. The immune response during acute HIV-1 infection: clues for vaccine development. Nat. Rev. Immunol. 10, 11–23 (2010).
Lee, J. et al. Molecular-level analysis of the serum antibody repertoire in young adults before and after seasonal influenza vaccination. Nat. Med. 22, 1456–1464 (2016).
Kaur, K. et al. High affinity antibodies against influenza characterize the plasmablast response in SLE patients after vaccination. PLoS One 10, e0125618 (2015).
Acknowledgements
The work of D.S.P. is supported by a Veterans Administration Merit Review grant and by a National Institutes for Health grant (1R01AR073935). The work of P.E.L. is supported by the RILITE Foundation.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks M. Aringer, M. Fritzler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pisetsky, D.S., Lipsky, P.E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol 16, 565–579 (2020). https://doi.org/10.1038/s41584-020-0480-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-020-0480-7
This article is cited by
-
Musculoskeletal symptoms in systemic lupus erythematosus patients and their impact on health-related quality of life
BMC Musculoskeletal Disorders (2024)
-
Molecular and structural basis of anti-DNA antibody specificity for pyrrolated proteins
Communications Biology (2024)
-
Cognitive deficits associated with novel intrathecal anti-nuclear antibodies
Molecular Psychiatry (2024)
-
Serum components influence antibody reactivity to glycan and DNA antigens
Scientific Reports (2023)
-
Pathogenesis of autoimmune disease
Nature Reviews Nephrology (2023)