-
PDF
- Split View
-
Views
-
Cite
Cite
W.L. Ng, C.M. Chu, A.K.L. Wu, V.C.C. Cheng, K.Y. Yuen, Lymphopenia at presentation is associated with increased risk of infections in patients with systemic lupus erythematosus, QJM: An International Journal of Medicine, Volume 99, Issue 1, January 2006, Pages 37–47, https://doi.org/10.1093/qjmed/hci155
Close - Share Icon Share
Abstract
Background: Patients with systemic lupus erythematosus (SLE) frequently suffer from infections, but the predisposing risk factors, as well as the exact frequency and nature of such infections, are not fully understood.
Aim: To describe the frequency, types and risk factors for infections in a group of Chinese patients in the early stage of SLE in Hong Kong.
Design: Retrospective record study.
Methods: We reviewed the case records of 91 Chinese SLE patients, presenting <12 months after SLE diagnosis. Details of major infections (requiring intravenous antimicrobial therapy, or any confirmed mycobacterial infection) and minor infections were reviewed. Clinical and laboratory features, the systemic lupus erythematosus disease activity index (SLEDAI) at presentation and drug treatment were recorded and analysed.
Results: There were 48 major infections and 62 minor infections during 260 patient-years of follow-up. A lymphocyte count ⩽1.0 × 109/l at presentation was independently associated with an increased risk for major infection: hazard ratio 4.7 (95%CI 1.6–13.7), p = 0.005. SLEDAI, use of corticosteroids and immunosuppressive therapy were all not associated with increased risk of infection.
Discussion: Lymphopenia was an important risk factor for major infections in this group of Chinese patients in the early stages of SLE. SLE patients with lymphopenia at presentation should be closely monitored for the development of infective complications.
Introduction
Infection is a major cause of mortality and morbidity in patients with systemic lupus erythematosus (SLE).1 In most modern series, infection ranks first or second as the commonest cause of death in SLE patients worldwide, including here in Hong Kong.2–4 Infection is also a major reason for hospital admissions in SLE patients.1
There are 5- and 10-fold increases in the rate of infection in SLE patients, compared to similar patients with nephrotic syndrome and rheumatoid arthritis, respectively.5 The increase in infection reflects the multiple immune defects associated with the disease.6 In addition, the use of corticosteroids and immunosuppressive drugs such as azathioprine and cyclophosphamide may lead to further disturbance of the immune system.
Several studies have attempted to identify risk factors for infection in SLE, with variable results.7,8 Major clinical predictors identified include active lupus,9–11 renal insufficiency12,13proteinuria.14 Use of corticosteroids at doses >20–60 mg/day has been reported to increase the risk of infection,9,10,12,13 as has previous use of steroids.15 However, in other studies, infections were independent of the amount of steroids used.14,16 Other risk factors, such as the use of pulse methylprednisolone11,14 or cyclophosphamide,10,11 have also been implicated. Interestingly, leukopenia has not been shown to be a risk factor for infection,9,10 a finding noted as surprising in a recent review article by Petri.1 However, no study to date has looked specifically into the effect of lymphopenia on the risk of infection in SLE.
One of the main drawbacks in the design of many of the aforementioned studies10,16 is that infection has been regarded as a dichotomous variable, and predictive risk factors identified by analysing differences between the groups with and without infections. In other studies,9,11 the incidence rate of infection was compared between groups with differing variables (e.g. steroid dosages or immunosuppressive therapy). However, these approaches do not take into account of the fact that the occurrence of infection is a time-dependent function, and infections may not occur at a uniform rate during the course of SLE. A more meaningful approach would be to analyse the occurrence of infection in the form of a survival study,17 where baseline characteristics are examined for their effects on the subsequent probability of surviving without infection.
The current study was conducted to investigate the frequency and nature of infections in a group of Chinese SLE patients in the early stages of the disease who were followed-up in a single rheumatology centre. Clinical and laboratory features at presentation of the disease (particularly lymphopenia) were examined for their effect on the probability of the subsequent occurrence of major infections. Only patients with disease duration <12 months were included, to identify risk factors for infection operating in the early stages of the disease, and to enhance uniformity in the care and follow up of the patients.
Methods
Patients
The Division of Rheumatology in the United Christian Hospital is the only rheumatology referral centre serving a population of approximately 0.7 million in the East Kowloon region of Hong Kong. All patients were followed-up by one of the authors (WLN). Patients were seen at regular intervals of 4–12 weeks, as guided by their clinical conditions. Blood tests were routinely performed for haematological, biochemical and serological profiles.
Between July 1994 and June 2000, 149 patients with SLE were seen on at least two occasions in the rheumatology clinic. All patients fulfilled the 1982 revised American College of Rheumatology (ACR) classification criteria for SLE.18 Fifty-eight patients were excluded from the study: 54 had had their SLE diagnosis for >1 year before their first presentation to the rheumatology clinic, two were non-Chinese, and two had the majority of their care in other hospitals, with insufficient information for analysis. The final study group thus comprised 91 patients. As we decided to assess the role of lymphocyte count as a predictor for infection, 82 patients with a lymphocyte count available at presentation were selected for risk factor analysis.
Data
Medical records were available for all the patients. Records were retrospectively reviewed using specifically designed forms. Background information was recorded for sex, age at diagnosis, duration of the disease at first presentation and duration of follow-up from time of diagnosis to last follow-up visit, death or end of the study period. The date of diagnosis was defined as the time when the patient first satisfied the 1982 revised criteria for the classification of SLE. The clinical features and laboratory results were examined in detail. Features of lupus corresponding to the ACR criteria were recorded. Neurological disorders were defined by the recently published ACR nomenclature and case definitions for neuropsychiatric lupus syndromes.19 Treatment charts were reviewed. The dosage of steroid used was recorded, expressed in milligrams of prednisolone or equivalent. The average dose of steroid used in the month preceding the first major infection was computed. The cumulative steroid dose was summed up to the time of occurrence of the first major infection or to the end of the follow-up, in those with and without major infection, respectively. This represented the period at which a patient was susceptible to the occurrence of the first major infection. The cumulative use of intravenous pulse methylprednisolone, azathioprine and other cytotoxic therapies, including cyclophosphamide, methotrexate and cyclosporin A, were recorded using a similar method. Disease activity of SLE at the time of diagnosis was retrospectively evaluated using the SLE Disease Activity Index (SLEDAI),20 a reliable and valid instrument for assessing lupus disease activity.21 Retrospective medical record abstraction for SLEDAI is a valid representation of lupus disease activity, with good intra-rater and inter-rater reliability.22
All non-scheduled admissions of SLE patients in the cohort within the 6-year period were recorded. The principal reason for admission was determined retrospectively at the time of the review. There were a small number of episodes in which the patients were admitted to other hospitals in Hong Kong. To ensure maximal capture of relevant information, a territory-wide search was performed via the Clinical Management System (CMS). The CMS is a computerized system that enables the retrieval of clinical and laboratory data in the majority of public hospitals in Hong Kong. To supplement any missing information, all discharge summaries, laboratory results and referral letters from other hospitals were reviewed, with special attention to the occurrence of infections.
All episodes of infection from the time of diagnosis of SLE were recorded. Infections were documented by clinical evidence and significant growth of pathogens from normally sterile sites. Alternatively, when no organism was found, the diagnosis was based on compatible clinical, radiological and laboratory features and/or a response to anti-microbial therapy in the absence of an alternative explanation. Major infections were defined as those requiring intravenous anti-microbial therapy. All infections by mycobacteria were regarded as major infections. Minor infections were defined as those that could be managed with oral or topical therapy. All cutaneous herpetic infections were classified as minor unless there was evidence of dissemination.
The incidence of infection was expressed as the number of infections per 100 patient years of follow-up, as previously described.9,11 The time interval from diagnosis of SLE to the first major infection was recorded and used for the analysis of infection-free survival. Potential risk factors for infection to be analysed included: (i) baseline demographic data (age, sex, duration of SLE on presentation); (ii) presence of various clinical features at disease presentation as described in the ACR criteria (malar rash, discoid rash, photosensitivity, oral ulcers, arthritis, serositis, renal disorders, neurologic disorders, haematological disorders and immunological disorders); and (iii) SLEDAI at the time of diagnosis of SLE. In particular, the effect of a lymphocyte count ⩽1.0 × 109/l at presentation of SLE was examined.
Both the activity of SLE23 and the lymphocyte count24,25 may vary during the course of illness, so their values at disease presentation may not be extrapolatable to the later part of the disease. To circumvent this problem, infection-free survival was re-analysed using follow-up data censored at 12 months. Furthermore, we re-examined the data by re-classifying major infections as those severe infections in which there was positive identification of micro-organisms.
Statistical analysis
Data are expressed as means and standard deviations (SD) for normally distributed measurements, and medians for non-parametric data. Continuous data between two groups was compared using the unpaired Student's t test or Mann-Whitney U test, as appropriate. Categorical data were compared using the χ2 test with Yates’ continuity correction, or Fisher's Exact Test, as appropriate. Analysis of duration of infection-free survival used the Kaplan-Meier plot. Univariate analysis of infection-free survival with respect to categorical and continuous variables used the log rank test and the Cox proportional hazard model, respectively. Variables with p < 0.1 were put into multivariate analysis, using the Cox proportional hazard model with stepwise selection. Factors with a two-tailed p value <0.05 in the final model were reported as independent variables for infection-free survival.
Results
Patient characteristics
There were 85 females and six males (F:M 14.2:1). Mean age at diagnosis was 37.2 ± 16.2 years (median 36, range 9–89). Mean duration of follow-up of the patients was 34.3 ± 25.0 months (median 28, range 1–84). Total duration of follow-up for the 91 eligible patients was 260 patient-years.
Table 1 summarizes the clinical features of the 91 patients in the cohort. The lymphocyte count at the time of diagnosis was available for 82 patients (90.1%). The mean value was 1.2 ± 0.9 × 109/l (median 1.1, range 0.2–5.1); 62% had a lymphocyte count <1.5 × 109/l and 49% a count of ⩽1.0 × 109/l. Mean SLEDAI at diagnosis was 8.2 ± 5.0 (median 7, range 0–24).
Presenting features and the cumulative occurrence of ACR criteria in the SLE cohort (n = 91)
| Feature . | Presentation . | Cumulative . | ||
|---|---|---|---|---|
| Clinical | ||||
| Malar rash | 38 (42%) | 38 (42%) | ||
| Discoid lesions | 3 (3%) | 3 (3%) | ||
| Photosensitivity | 23 (25%) | 25 (28%) | ||
| Oral ulcers | 8 (9%) | 8 (9%) | ||
| Arthritis | 63 (69%) | 63 (69%) | ||
| Serositis | 14 (15%) | 14 (15%) | ||
| Renal disordera | 25 (27%) | 27 (30%) | ||
| All neuropsychiatricb | 5 (6%) | 5 (6%) | ||
| Haematological | ||||
| Haemolytic anaemia | 14 (15%) | 15 (17%) | ||
| Leukopenia (<4 × 109/l) | 25 (27%) | 40 (44%) | ||
| Lymphopenia (<1.5 × 109/l) | 51/82 (62%) | 81/90 (90%) | ||
| Lymphopenia (⩽1.0 × 109/l) | 40/82 (49%) | 59/90 (66%) | ||
| Thrombocytopenia (<100 × 109/l) | 6 (7%) | 11 (12%) | ||
| Serological | ||||
| Anti-nuclear antibody | 91 (100) | NA | ||
| Anti-ds DNA or Anti-SM antibodies | 76 (83.5) | NA | ||
| Feature . | Presentation . | Cumulative . | ||
|---|---|---|---|---|
| Clinical | ||||
| Malar rash | 38 (42%) | 38 (42%) | ||
| Discoid lesions | 3 (3%) | 3 (3%) | ||
| Photosensitivity | 23 (25%) | 25 (28%) | ||
| Oral ulcers | 8 (9%) | 8 (9%) | ||
| Arthritis | 63 (69%) | 63 (69%) | ||
| Serositis | 14 (15%) | 14 (15%) | ||
| Renal disordera | 25 (27%) | 27 (30%) | ||
| All neuropsychiatricb | 5 (6%) | 5 (6%) | ||
| Haematological | ||||
| Haemolytic anaemia | 14 (15%) | 15 (17%) | ||
| Leukopenia (<4 × 109/l) | 25 (27%) | 40 (44%) | ||
| Lymphopenia (<1.5 × 109/l) | 51/82 (62%) | 81/90 (90%) | ||
| Lymphopenia (⩽1.0 × 109/l) | 40/82 (49%) | 59/90 (66%) | ||
| Thrombocytopenia (<100 × 109/l) | 6 (7%) | 11 (12%) | ||
| Serological | ||||
| Anti-nuclear antibody | 91 (100) | NA | ||
| Anti-ds DNA or Anti-SM antibodies | 76 (83.5) | NA | ||
aPersistent proteinuria >0.5 g/day or presence of cellular casts. bSeizures (2), transverse myelitis (1), subarachnoid haemorrhage (1) and peripheral neuropathy (1). NA, not applicable.
Presenting features and the cumulative occurrence of ACR criteria in the SLE cohort (n = 91)
| Feature . | Presentation . | Cumulative . | ||
|---|---|---|---|---|
| Clinical | ||||
| Malar rash | 38 (42%) | 38 (42%) | ||
| Discoid lesions | 3 (3%) | 3 (3%) | ||
| Photosensitivity | 23 (25%) | 25 (28%) | ||
| Oral ulcers | 8 (9%) | 8 (9%) | ||
| Arthritis | 63 (69%) | 63 (69%) | ||
| Serositis | 14 (15%) | 14 (15%) | ||
| Renal disordera | 25 (27%) | 27 (30%) | ||
| All neuropsychiatricb | 5 (6%) | 5 (6%) | ||
| Haematological | ||||
| Haemolytic anaemia | 14 (15%) | 15 (17%) | ||
| Leukopenia (<4 × 109/l) | 25 (27%) | 40 (44%) | ||
| Lymphopenia (<1.5 × 109/l) | 51/82 (62%) | 81/90 (90%) | ||
| Lymphopenia (⩽1.0 × 109/l) | 40/82 (49%) | 59/90 (66%) | ||
| Thrombocytopenia (<100 × 109/l) | 6 (7%) | 11 (12%) | ||
| Serological | ||||
| Anti-nuclear antibody | 91 (100) | NA | ||
| Anti-ds DNA or Anti-SM antibodies | 76 (83.5) | NA | ||
| Feature . | Presentation . | Cumulative . | ||
|---|---|---|---|---|
| Clinical | ||||
| Malar rash | 38 (42%) | 38 (42%) | ||
| Discoid lesions | 3 (3%) | 3 (3%) | ||
| Photosensitivity | 23 (25%) | 25 (28%) | ||
| Oral ulcers | 8 (9%) | 8 (9%) | ||
| Arthritis | 63 (69%) | 63 (69%) | ||
| Serositis | 14 (15%) | 14 (15%) | ||
| Renal disordera | 25 (27%) | 27 (30%) | ||
| All neuropsychiatricb | 5 (6%) | 5 (6%) | ||
| Haematological | ||||
| Haemolytic anaemia | 14 (15%) | 15 (17%) | ||
| Leukopenia (<4 × 109/l) | 25 (27%) | 40 (44%) | ||
| Lymphopenia (<1.5 × 109/l) | 51/82 (62%) | 81/90 (90%) | ||
| Lymphopenia (⩽1.0 × 109/l) | 40/82 (49%) | 59/90 (66%) | ||
| Thrombocytopenia (<100 × 109/l) | 6 (7%) | 11 (12%) | ||
| Serological | ||||
| Anti-nuclear antibody | 91 (100) | NA | ||
| Anti-ds DNA or Anti-SM antibodies | 76 (83.5) | NA | ||
aPersistent proteinuria >0.5 g/day or presence of cellular casts. bSeizures (2), transverse myelitis (1), subarachnoid haemorrhage (1) and peripheral neuropathy (1). NA, not applicable.
Infections and hospital admissions
Forty-eight episodes of major infections occurred in 27 patients (29.7%). Seven (25.9%) episodes occurred at disease presentation, and 63% within the first year after diagnosis of SLE. The majority of patients (66.7%) had a single infection (Table 2). Details of the infections were shown in Table 3. Micro-organisms could be identified in 30 episodes (62.5%). The overall incidence was 18.5 infections per 100 patient-years of follow-up. A total of 62 episodes of minor infections occurred in 37 patients (40.7%) (Table 4), representing an incidence of 23.8 infections per 100 patient-years.
Frequencies of major infection (n = 27)
| No. of major infections . | Patients . |
|---|---|
| 1 | 18 (67%) |
| 2 | 6 (22%) |
| 3 | 2 (7%) |
| 11 | 1* (4%) |
| No. of major infections . | Patients . |
|---|---|
| 1 | 18 (67%) |
| 2 | 6 (22%) |
| 3 | 2 (7%) |
| 11 | 1* (4%) |
*A 31-year-old woman who presented with transverse myelitis and status epilepticus requiring admission to intensive care unit. She developed nosocomial pneumonia and recurrent catheter-related urinary tract infection.
Frequencies of major infection (n = 27)
| No. of major infections . | Patients . |
|---|---|
| 1 | 18 (67%) |
| 2 | 6 (22%) |
| 3 | 2 (7%) |
| 11 | 1* (4%) |
| No. of major infections . | Patients . |
|---|---|
| 1 | 18 (67%) |
| 2 | 6 (22%) |
| 3 | 2 (7%) |
| 11 | 1* (4%) |
*A 31-year-old woman who presented with transverse myelitis and status epilepticus requiring admission to intensive care unit. She developed nosocomial pneumonia and recurrent catheter-related urinary tract infection.
Details of major infections (n = 27)
| Site (no. of episodes) . | Organism . | n (%) . |
|---|---|---|
| Pneumonia (16) | Methicillin-resistant Staphylococcus aureus | 3 (6.3%) |
| Mycobacterium tuberculosis | 2 (4.2%) | |
| Pseudomonas aeruginosa | 1 (2.0%) | |
| Stenotrophomonas maltophila | 1 (2.0%) | |
| Haemophilus influenzae | 1 (2.0%) | |
| Haemophilus parainfluenzae | 1 (2.0%) | |
| No organism identified | 7 (14.6%) | |
| Urinary tract (13) | E. coli | 10 (20.8%) |
| Pseudomonas aeruginosa | 1 (2.0%) | |
| Klebsiella spp | 1 (2.0%) | |
| No organism identified | 1 (2.0%) | |
| Septicaemia and disseminated infection (5) | Mycobacterium tuberculosis | 2 (4.2%) |
| Listeria monocytogenes | 1 (2.0%) | |
| No organism identified | 2 (4.2%) | |
| Cutaneous (5) | Staphylococcus aureus | 2 (4.2%) |
| Mycobacterium tuberculosis | 1 (2.0%) | |
| No organism identified | 2 (4.2%) | |
| Lymphadenitis (4) | Mycobacterium tuberculosis | 2 (4.2%) |
| No organism identified | 2 (4.2%) | |
| Upper respiratory tract (3) | No organism identified | 3 (6.3%) |
| Septic arthritis (1) | Staphylococcus aureus | 1 (2.0%) |
| Meningitis (1) | No organism identified | 1 (2.0%) |
| Total (48) | 48 (100%) |
| Site (no. of episodes) . | Organism . | n (%) . |
|---|---|---|
| Pneumonia (16) | Methicillin-resistant Staphylococcus aureus | 3 (6.3%) |
| Mycobacterium tuberculosis | 2 (4.2%) | |
| Pseudomonas aeruginosa | 1 (2.0%) | |
| Stenotrophomonas maltophila | 1 (2.0%) | |
| Haemophilus influenzae | 1 (2.0%) | |
| Haemophilus parainfluenzae | 1 (2.0%) | |
| No organism identified | 7 (14.6%) | |
| Urinary tract (13) | E. coli | 10 (20.8%) |
| Pseudomonas aeruginosa | 1 (2.0%) | |
| Klebsiella spp | 1 (2.0%) | |
| No organism identified | 1 (2.0%) | |
| Septicaemia and disseminated infection (5) | Mycobacterium tuberculosis | 2 (4.2%) |
| Listeria monocytogenes | 1 (2.0%) | |
| No organism identified | 2 (4.2%) | |
| Cutaneous (5) | Staphylococcus aureus | 2 (4.2%) |
| Mycobacterium tuberculosis | 1 (2.0%) | |
| No organism identified | 2 (4.2%) | |
| Lymphadenitis (4) | Mycobacterium tuberculosis | 2 (4.2%) |
| No organism identified | 2 (4.2%) | |
| Upper respiratory tract (3) | No organism identified | 3 (6.3%) |
| Septic arthritis (1) | Staphylococcus aureus | 1 (2.0%) |
| Meningitis (1) | No organism identified | 1 (2.0%) |
| Total (48) | 48 (100%) |
Details of major infections (n = 27)
| Site (no. of episodes) . | Organism . | n (%) . |
|---|---|---|
| Pneumonia (16) | Methicillin-resistant Staphylococcus aureus | 3 (6.3%) |
| Mycobacterium tuberculosis | 2 (4.2%) | |
| Pseudomonas aeruginosa | 1 (2.0%) | |
| Stenotrophomonas maltophila | 1 (2.0%) | |
| Haemophilus influenzae | 1 (2.0%) | |
| Haemophilus parainfluenzae | 1 (2.0%) | |
| No organism identified | 7 (14.6%) | |
| Urinary tract (13) | E. coli | 10 (20.8%) |
| Pseudomonas aeruginosa | 1 (2.0%) | |
| Klebsiella spp | 1 (2.0%) | |
| No organism identified | 1 (2.0%) | |
| Septicaemia and disseminated infection (5) | Mycobacterium tuberculosis | 2 (4.2%) |
| Listeria monocytogenes | 1 (2.0%) | |
| No organism identified | 2 (4.2%) | |
| Cutaneous (5) | Staphylococcus aureus | 2 (4.2%) |
| Mycobacterium tuberculosis | 1 (2.0%) | |
| No organism identified | 2 (4.2%) | |
| Lymphadenitis (4) | Mycobacterium tuberculosis | 2 (4.2%) |
| No organism identified | 2 (4.2%) | |
| Upper respiratory tract (3) | No organism identified | 3 (6.3%) |
| Septic arthritis (1) | Staphylococcus aureus | 1 (2.0%) |
| Meningitis (1) | No organism identified | 1 (2.0%) |
| Total (48) | 48 (100%) |
| Site (no. of episodes) . | Organism . | n (%) . |
|---|---|---|
| Pneumonia (16) | Methicillin-resistant Staphylococcus aureus | 3 (6.3%) |
| Mycobacterium tuberculosis | 2 (4.2%) | |
| Pseudomonas aeruginosa | 1 (2.0%) | |
| Stenotrophomonas maltophila | 1 (2.0%) | |
| Haemophilus influenzae | 1 (2.0%) | |
| Haemophilus parainfluenzae | 1 (2.0%) | |
| No organism identified | 7 (14.6%) | |
| Urinary tract (13) | E. coli | 10 (20.8%) |
| Pseudomonas aeruginosa | 1 (2.0%) | |
| Klebsiella spp | 1 (2.0%) | |
| No organism identified | 1 (2.0%) | |
| Septicaemia and disseminated infection (5) | Mycobacterium tuberculosis | 2 (4.2%) |
| Listeria monocytogenes | 1 (2.0%) | |
| No organism identified | 2 (4.2%) | |
| Cutaneous (5) | Staphylococcus aureus | 2 (4.2%) |
| Mycobacterium tuberculosis | 1 (2.0%) | |
| No organism identified | 2 (4.2%) | |
| Lymphadenitis (4) | Mycobacterium tuberculosis | 2 (4.2%) |
| No organism identified | 2 (4.2%) | |
| Upper respiratory tract (3) | No organism identified | 3 (6.3%) |
| Septic arthritis (1) | Staphylococcus aureus | 1 (2.0%) |
| Meningitis (1) | No organism identified | 1 (2.0%) |
| Total (48) | 48 (100%) |
Minor episodes of infections in 37 patients
| Site (no. of episodes) . | Organism . | n (%) . |
|---|---|---|
| Upper respiratory tract (25) | Haemophilus influenzae | 3 (4.8) |
| Haemophilus parainfluenzae | 2 (3.2) | |
| Influenza A | 1 (1.6) | |
| No organism identified | 20 (32.2) | |
| Cutaneous (18) | Herpes zoster | 11 (17.7) |
| Staphylococcus aureus | 1 (1.6) | |
| Mixed growth (Morganella morganii, Klebsiella pneumoniae) | 1 (1.6) | |
| No organism identified | 5 (8.1) | |
| Urinary (9) | E. coli | 5 (8.1) |
| Enterococcus faecalis | 1 (1.6) | |
| Strepotococcus agalactiae | 1 (1.6) | |
| Group D streptococcus | 1 (1.6) | |
| Pseudomonas aeruginosa | 1 (1.6) | |
| Lymphadenitis (6) | No organism identified | 6 (7.3) |
| Vaginal (1) | Gardnerella vaginalis | 1 (1.6) |
| Sinusitis (1) | Streptococcus milleri | 1 (1.6) |
| Keratitis (1) | Herpes simplex | 1 (1.6) |
| Total (62) | 62 (100) |
| Site (no. of episodes) . | Organism . | n (%) . |
|---|---|---|
| Upper respiratory tract (25) | Haemophilus influenzae | 3 (4.8) |
| Haemophilus parainfluenzae | 2 (3.2) | |
| Influenza A | 1 (1.6) | |
| No organism identified | 20 (32.2) | |
| Cutaneous (18) | Herpes zoster | 11 (17.7) |
| Staphylococcus aureus | 1 (1.6) | |
| Mixed growth (Morganella morganii, Klebsiella pneumoniae) | 1 (1.6) | |
| No organism identified | 5 (8.1) | |
| Urinary (9) | E. coli | 5 (8.1) |
| Enterococcus faecalis | 1 (1.6) | |
| Strepotococcus agalactiae | 1 (1.6) | |
| Group D streptococcus | 1 (1.6) | |
| Pseudomonas aeruginosa | 1 (1.6) | |
| Lymphadenitis (6) | No organism identified | 6 (7.3) |
| Vaginal (1) | Gardnerella vaginalis | 1 (1.6) |
| Sinusitis (1) | Streptococcus milleri | 1 (1.6) |
| Keratitis (1) | Herpes simplex | 1 (1.6) |
| Total (62) | 62 (100) |
Minor episodes of infections in 37 patients
| Site (no. of episodes) . | Organism . | n (%) . |
|---|---|---|
| Upper respiratory tract (25) | Haemophilus influenzae | 3 (4.8) |
| Haemophilus parainfluenzae | 2 (3.2) | |
| Influenza A | 1 (1.6) | |
| No organism identified | 20 (32.2) | |
| Cutaneous (18) | Herpes zoster | 11 (17.7) |
| Staphylococcus aureus | 1 (1.6) | |
| Mixed growth (Morganella morganii, Klebsiella pneumoniae) | 1 (1.6) | |
| No organism identified | 5 (8.1) | |
| Urinary (9) | E. coli | 5 (8.1) |
| Enterococcus faecalis | 1 (1.6) | |
| Strepotococcus agalactiae | 1 (1.6) | |
| Group D streptococcus | 1 (1.6) | |
| Pseudomonas aeruginosa | 1 (1.6) | |
| Lymphadenitis (6) | No organism identified | 6 (7.3) |
| Vaginal (1) | Gardnerella vaginalis | 1 (1.6) |
| Sinusitis (1) | Streptococcus milleri | 1 (1.6) |
| Keratitis (1) | Herpes simplex | 1 (1.6) |
| Total (62) | 62 (100) |
| Site (no. of episodes) . | Organism . | n (%) . |
|---|---|---|
| Upper respiratory tract (25) | Haemophilus influenzae | 3 (4.8) |
| Haemophilus parainfluenzae | 2 (3.2) | |
| Influenza A | 1 (1.6) | |
| No organism identified | 20 (32.2) | |
| Cutaneous (18) | Herpes zoster | 11 (17.7) |
| Staphylococcus aureus | 1 (1.6) | |
| Mixed growth (Morganella morganii, Klebsiella pneumoniae) | 1 (1.6) | |
| No organism identified | 5 (8.1) | |
| Urinary (9) | E. coli | 5 (8.1) |
| Enterococcus faecalis | 1 (1.6) | |
| Strepotococcus agalactiae | 1 (1.6) | |
| Group D streptococcus | 1 (1.6) | |
| Pseudomonas aeruginosa | 1 (1.6) | |
| Lymphadenitis (6) | No organism identified | 6 (7.3) |
| Vaginal (1) | Gardnerella vaginalis | 1 (1.6) |
| Sinusitis (1) | Streptococcus milleri | 1 (1.6) |
| Keratitis (1) | Herpes simplex | 1 (1.6) |
| Total (62) | 62 (100) |
There were 118 non-scheduled hospital admissions in 51 (56.0%) patients during the study period. Active SLE accounted for 56 (47.5%), infections for 41 (34.7%), and the remaining 15 (12.7%) were due to treatment complications and other causes unrelated to SLE.
Risk factors for infection
The 82 patients with lymphocyte counts available at the time of presentation were included in the risk factor analysis (Table 5). The first group developed at least one major infection during their clinical course; the second group never developed any major infection. The two groups were similar with regard to age, sex distribution, duration of follow-up, presence of leukopenia, thrombocytopenia, renal and neurological disorders and prevalence of anti-DNA antibody at presentation. Other ACR clinical features were also similar (not shown).
Demographic and clinical profile of 82 SLE patients (with lymphocyte count available at presentation) with and without major infections
| . | Patients with major infection (n = 27) . | Patients without major infection (n = 55) . |
|---|---|---|
| Age at diagnosis (years) | 32.0 (14–80) | 37 (9–89) |
| Total follow-up (months) | 28 (1–76) | 27 (1–84) |
| Female sex (%) | 25 (92.7) | 51 (92.7) |
| Leukopenia <4 × 109/l (%) | 13 (48.1%) | 27 (49.1%) |
| Haemolytic anaemia (%) | 10 (37.0%) | 5 (9.1%) |
| Thrombocytopenia <100 × 109/l (%) | 5 (18.5%) | 4 (7.3%) |
| Renal diseasea (%) | 10 (37.0%) | 14 (25.5%) |
| Neurological diseaseb (%) | 3 (11.1%) | 20 (3.6%) |
| Positive anti-dsDNA (%) | 20 (81.5%) | 45 (81.8%) |
| Cumulative steroid dosec in mg | 615d (0–17651) | 1568d (0–30767) |
| Pulse i.v. methylprednisolone (%) | 2 (7.4%) | 5 (9.1%) |
| Azathioprine (%) | 2 (7.4%)e5 (18.5%)f | 16 (29.1%)f |
| Cytotoxic therapyg (%) | 2 (7.4%)e, 5 (18.5%)f | 7 (12.7%) |
| SLEDAI at time of diagnosis | 9 (3–24) | 6 (0–18) |
| Lymphocyte count at presentation (× 109/l) (mean ± SD) | 0.92 ± 0.9 | 1.36 ± 0.8 |
| . | Patients with major infection (n = 27) . | Patients without major infection (n = 55) . |
|---|---|---|
| Age at diagnosis (years) | 32.0 (14–80) | 37 (9–89) |
| Total follow-up (months) | 28 (1–76) | 27 (1–84) |
| Female sex (%) | 25 (92.7) | 51 (92.7) |
| Leukopenia <4 × 109/l (%) | 13 (48.1%) | 27 (49.1%) |
| Haemolytic anaemia (%) | 10 (37.0%) | 5 (9.1%) |
| Thrombocytopenia <100 × 109/l (%) | 5 (18.5%) | 4 (7.3%) |
| Renal diseasea (%) | 10 (37.0%) | 14 (25.5%) |
| Neurological diseaseb (%) | 3 (11.1%) | 20 (3.6%) |
| Positive anti-dsDNA (%) | 20 (81.5%) | 45 (81.8%) |
| Cumulative steroid dosec in mg | 615d (0–17651) | 1568d (0–30767) |
| Pulse i.v. methylprednisolone (%) | 2 (7.4%) | 5 (9.1%) |
| Azathioprine (%) | 2 (7.4%)e5 (18.5%)f | 16 (29.1%)f |
| Cytotoxic therapyg (%) | 2 (7.4%)e, 5 (18.5%)f | 7 (12.7%) |
| SLEDAI at time of diagnosis | 9 (3–24) | 6 (0–18) |
| Lymphocyte count at presentation (× 109/l) (mean ± SD) | 0.92 ± 0.9 | 1.36 ± 0.8 |
Data are medians (range) unless otherwise stated. aDefined as persistent proteinuria >0.5 g/day or presence of cellular casts. bIncluded the following: seizures (2), transverse myelitis (1), subarachnoid haemorrhage (1) and peripheral neuropathy (1). cDosages expressed as mg of prednisolone or equivalent. dCumulative dose of steroid received was summed up to the time of occurrence of the first major infection and to the end of follow-up, in the groups with and without major infection, respectively. eCumulative use before the occurrence of the first major infection. fCumulative use at the end of follow-up. gIncluded use of cyclophosphamide, methotrexate or cyclosporin A.
Demographic and clinical profile of 82 SLE patients (with lymphocyte count available at presentation) with and without major infections
| . | Patients with major infection (n = 27) . | Patients without major infection (n = 55) . |
|---|---|---|
| Age at diagnosis (years) | 32.0 (14–80) | 37 (9–89) |
| Total follow-up (months) | 28 (1–76) | 27 (1–84) |
| Female sex (%) | 25 (92.7) | 51 (92.7) |
| Leukopenia <4 × 109/l (%) | 13 (48.1%) | 27 (49.1%) |
| Haemolytic anaemia (%) | 10 (37.0%) | 5 (9.1%) |
| Thrombocytopenia <100 × 109/l (%) | 5 (18.5%) | 4 (7.3%) |
| Renal diseasea (%) | 10 (37.0%) | 14 (25.5%) |
| Neurological diseaseb (%) | 3 (11.1%) | 20 (3.6%) |
| Positive anti-dsDNA (%) | 20 (81.5%) | 45 (81.8%) |
| Cumulative steroid dosec in mg | 615d (0–17651) | 1568d (0–30767) |
| Pulse i.v. methylprednisolone (%) | 2 (7.4%) | 5 (9.1%) |
| Azathioprine (%) | 2 (7.4%)e5 (18.5%)f | 16 (29.1%)f |
| Cytotoxic therapyg (%) | 2 (7.4%)e, 5 (18.5%)f | 7 (12.7%) |
| SLEDAI at time of diagnosis | 9 (3–24) | 6 (0–18) |
| Lymphocyte count at presentation (× 109/l) (mean ± SD) | 0.92 ± 0.9 | 1.36 ± 0.8 |
| . | Patients with major infection (n = 27) . | Patients without major infection (n = 55) . |
|---|---|---|
| Age at diagnosis (years) | 32.0 (14–80) | 37 (9–89) |
| Total follow-up (months) | 28 (1–76) | 27 (1–84) |
| Female sex (%) | 25 (92.7) | 51 (92.7) |
| Leukopenia <4 × 109/l (%) | 13 (48.1%) | 27 (49.1%) |
| Haemolytic anaemia (%) | 10 (37.0%) | 5 (9.1%) |
| Thrombocytopenia <100 × 109/l (%) | 5 (18.5%) | 4 (7.3%) |
| Renal diseasea (%) | 10 (37.0%) | 14 (25.5%) |
| Neurological diseaseb (%) | 3 (11.1%) | 20 (3.6%) |
| Positive anti-dsDNA (%) | 20 (81.5%) | 45 (81.8%) |
| Cumulative steroid dosec in mg | 615d (0–17651) | 1568d (0–30767) |
| Pulse i.v. methylprednisolone (%) | 2 (7.4%) | 5 (9.1%) |
| Azathioprine (%) | 2 (7.4%)e5 (18.5%)f | 16 (29.1%)f |
| Cytotoxic therapyg (%) | 2 (7.4%)e, 5 (18.5%)f | 7 (12.7%) |
| SLEDAI at time of diagnosis | 9 (3–24) | 6 (0–18) |
| Lymphocyte count at presentation (× 109/l) (mean ± SD) | 0.92 ± 0.9 | 1.36 ± 0.8 |
Data are medians (range) unless otherwise stated. aDefined as persistent proteinuria >0.5 g/day or presence of cellular casts. bIncluded the following: seizures (2), transverse myelitis (1), subarachnoid haemorrhage (1) and peripheral neuropathy (1). cDosages expressed as mg of prednisolone or equivalent. dCumulative dose of steroid received was summed up to the time of occurrence of the first major infection and to the end of follow-up, in the groups with and without major infection, respectively. eCumulative use before the occurrence of the first major infection. fCumulative use at the end of follow-up. gIncluded use of cyclophosphamide, methotrexate or cyclosporin A.
With regard to steroid therapy, the cumulative steroid doses were similar in both groups. Of those with major infections, 9 (33.3%) did not receive any prior steroid therapy, and another 10 (37.0%) received an average dose of ⩽10 mg prednisolone in the month preceding the first major infection. The percentages of patients who had received intravenous pulse methylprednisolone, azathioprine or cytotoxic therapy were similar in the two groups.
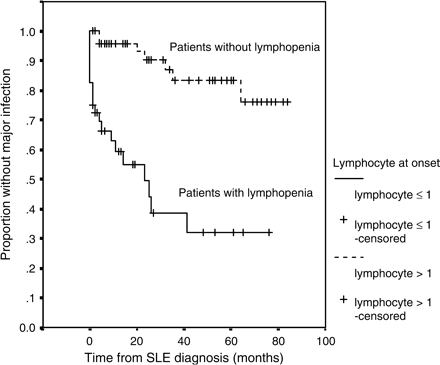
The time period from SLE diagnosis to the first major infection was analysed. Risk factors for infection-free survival were examined using demographic data such as sex, age, auto-antibodies, SLEDAI and prevalence of various clinical features. On univariate analysis by log-rank test, both the presence of haemolytic anaemia (p < 0.005) and lymphopenia ⩽1.0 × 109/l at time of SLE diagnosis (p < 0.0001) were associated with an increased risk of development of a major infection during follow-up (Table 6). Figure 1 shows the survival curves according to the presence or absence of lymphopenia ⩽1.0 × 109/l at diagnosis. On multivariate analysis using Cox regression, lymphopenia ⩽1.0 × 109/l at disease presentation was the only independent predictor for the occurrence of a major infection: hazard ratio (HR) 4.7 (95%CI 1.6–13.7), p = 0.005.
Analysis of potential risk factors associated with major infections in SLE
| . | Log-rank test p . | Unadjusted hazard ratio (95%CI) by univariate Cox's regression and p value . | Adjusted hazard ratio (95%CI) by multivariate Cox's regression and p value . |
|---|---|---|---|
| Lymphocytes ⩽1.0 × 109/l | <0.0001 | 5.0 (1.9–13.3) p < 0.001 | 4.7 (1.6–13.7) p = 0.005 |
| Presence of haemolytic anaemia | <0.005 | 2.5 (0.9–6.5) p = 0.066 | 1.2 (0.4–3.4) p = 0.78 |
| Male sex | 0.98 | 1.5 (0.2–11.2) p = 0.7 | – |
| Age (per year) | – | 1.02 (0.99–1.04) p = 0.28 | – |
| Positive anti-ds DNA | 0.67 | 1.1 (0.4–3.4) p = 0.82 | – |
| SLEDAI (per point) | – | 1.04 (0.95–1.15) p = 0.37 | – |
| . | Log-rank test p . | Unadjusted hazard ratio (95%CI) by univariate Cox's regression and p value . | Adjusted hazard ratio (95%CI) by multivariate Cox's regression and p value . |
|---|---|---|---|
| Lymphocytes ⩽1.0 × 109/l | <0.0001 | 5.0 (1.9–13.3) p < 0.001 | 4.7 (1.6–13.7) p = 0.005 |
| Presence of haemolytic anaemia | <0.005 | 2.5 (0.9–6.5) p = 0.066 | 1.2 (0.4–3.4) p = 0.78 |
| Male sex | 0.98 | 1.5 (0.2–11.2) p = 0.7 | – |
| Age (per year) | – | 1.02 (0.99–1.04) p = 0.28 | – |
| Positive anti-ds DNA | 0.67 | 1.1 (0.4–3.4) p = 0.82 | – |
| SLEDAI (per point) | – | 1.04 (0.95–1.15) p = 0.37 | – |
SLEDAI, systemic lupus erythematosus disease activity index.
Analysis of potential risk factors associated with major infections in SLE
| . | Log-rank test p . | Unadjusted hazard ratio (95%CI) by univariate Cox's regression and p value . | Adjusted hazard ratio (95%CI) by multivariate Cox's regression and p value . |
|---|---|---|---|
| Lymphocytes ⩽1.0 × 109/l | <0.0001 | 5.0 (1.9–13.3) p < 0.001 | 4.7 (1.6–13.7) p = 0.005 |
| Presence of haemolytic anaemia | <0.005 | 2.5 (0.9–6.5) p = 0.066 | 1.2 (0.4–3.4) p = 0.78 |
| Male sex | 0.98 | 1.5 (0.2–11.2) p = 0.7 | – |
| Age (per year) | – | 1.02 (0.99–1.04) p = 0.28 | – |
| Positive anti-ds DNA | 0.67 | 1.1 (0.4–3.4) p = 0.82 | – |
| SLEDAI (per point) | – | 1.04 (0.95–1.15) p = 0.37 | – |
| . | Log-rank test p . | Unadjusted hazard ratio (95%CI) by univariate Cox's regression and p value . | Adjusted hazard ratio (95%CI) by multivariate Cox's regression and p value . |
|---|---|---|---|
| Lymphocytes ⩽1.0 × 109/l | <0.0001 | 5.0 (1.9–13.3) p < 0.001 | 4.7 (1.6–13.7) p = 0.005 |
| Presence of haemolytic anaemia | <0.005 | 2.5 (0.9–6.5) p = 0.066 | 1.2 (0.4–3.4) p = 0.78 |
| Male sex | 0.98 | 1.5 (0.2–11.2) p = 0.7 | – |
| Age (per year) | – | 1.02 (0.99–1.04) p = 0.28 | – |
| Positive anti-ds DNA | 0.67 | 1.1 (0.4–3.4) p = 0.82 | – |
| SLEDAI (per point) | – | 1.04 (0.95–1.15) p = 0.37 | – |
SLEDAI, systemic lupus erythematosus disease activity index.
Cumulative probability of infection-free survival for the complete follow-up period in SLE patients with or without lymphopenia (⩽1.0 × 109/l) at presentation (n = 82).
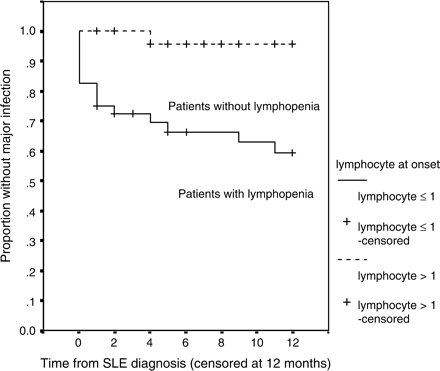
To examine further the predictive value of lymphopenia at disease onset, the data were re-analysed, with follow-up censored at 12 months. During the first 12-month period, there were 18 major infections. Sixteen (40%) of the 40 patients with a lymphocyte count ⩽1.0 × 109/l developed a major infection, compared to only 3 (4.8%) of the other 42 (Figure 2). Lymphopenia ⩽1.0 × 109/l at presentation of SLE remained the only independent risk factor predicting occurrence of major infection: HR 6.2 (95%CI 1.3–29.3), p = 0.021 by Cox regression.
Cumulative probability of infection-free survival for the initial 12 months of follow-up in SLE patients with or without lymphopenia (⩽1.0 × 109/l) at presentation (n = 82).
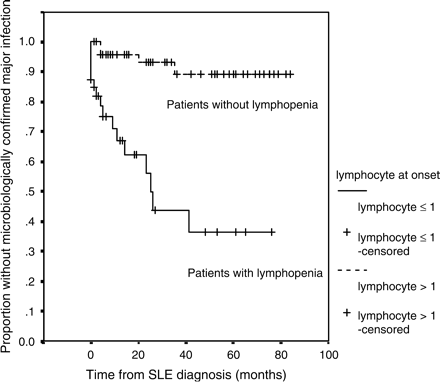
We also analysed the data by re-classifying major infections to include only those in which there was positive identification of the infecting micro-organism. Under this new definition, major infection occurred in 12 (30%) of the 40 patients with a lymphocyte count ⩽1.0 × 109/l vs. 3 (7.1%) of the 42 others (Figure 3). Lymphopenia ⩽1.0 × 109/l at time of diagnosis of SLE was still the only predictor for occurrence of major infection: HR 18.1 (95%CI 6.5–50.2), p < 0.001 by Cox regression.
Cumulative probability of infection-free survival (for microbiologically confirmed major infections) for the complete follow-up period in SLE patients with or without lymphopenia (⩽1.0 × 109/l) at presentation (n = 82).
Deaths
There was only one death in the study during the follow-up period: a 77-year-old lady with history of Evans’ syndrome (autoimmune haemolytic anaemia and thrombocytopenia) treated with steroids and splenectomy. She subsequently developed photosensitive rash, proteinuria with positive ANA and anti-DNA. Two years later, she developed ischaemic colitis requiring bowel resection, and died post-operatively from nosocomial pneumonia (methicillin-resistant Staphylococcus aureus). Lupus was not active at the time of death. No autopsy was performed.
Discussion
This is the first report from Hong Kong of the risk factors associated with infections in Chinese patients with SLE. The study was performed on ambulatory patients followed-up in a tertiary referral hospital, all seen within 12 months of diagnosis (i.e. patients in the early stages of SLE). All patients were followed in a single centre, under the care of one rheumatologist.
Infection was common in these patients, with an overall incidence rate of 18.5 per 100 patient-years of follow-up. In the study of Ginzler et al., the incidence of major infections was 13.9 per 100 patient years.9 Our infection rate was almost identical to that in a group of Malaysian SLE patients.11 but a much lower rate of 6.9 per 100 patient years was observed separately in Canadian26 and Swedish populations.27 Overall, 34.7% of the hospitalizations within the study period were related to infections, whereas 47.6% were attributed to SLE activity. In contrast, in the Hopkins Lupus Cohort, which included sicker patients with longer disease duration, infections were responsible for only 14% of admissions.28 In a large, multi-centre prospective study, infections accounted for 28.9% of deaths among a cohort of SLE patients, and was the second most common cause of death identified in that study.29
Most major infections in this cohort were caused by common bacterial or viral pathogens, most commonly involving the lungs and urinary tract. Escherichia coli and Staphyloccocus aureus were the leading causative pathogens. There was one case of Listeria monocytogenes bacteraemia, a classic intracellular organism requiring intact cell-mediated immunity for body defense.6 Krau et al. reported seven cases of listeriosis in SLE patients, all having severe lymphopenia and receiving immunosuppressive therapies.30 Of particular interest was the high rate (6.6%) of mycobacterial infection in our patients, similar to the prevalence of tuberculosis reported in 390 Philippino SLE patients (13.8% over 11 years).31 In a previous Hong Kong study, there were nine cases of tuberculosis among 156 SLE patients (5.8%) over 4 years.32 This may reflect the high local prevalence of tuberculosis in our community. In addition, eleven patients (12.1%) developed herpes zoster infection in this cohort, with no disseminated infection. Kahl et al. reported a similar prevalence of 13.5% among 348 SLE patients.33
Severe opportunistic infections by organisms such as Nocardia, Pneumocystis carinii, Cytomegalovirus and systemic fungal infections were not seen in this cohort. It was unlikely that such infections were missed, as they would have fatal outcomes if left untreated.34 There could be a number of reasons for the absence of severe opportunistic infections in our study. It is possible that patients with more severe infections at presentation might have succumbed before they could be recruited into our study. Severe opportunistic infections were strongly associated with use of immunosuppressants,34–36 and patients with longer disease duration and more extensive use of immunosuppressants were under-represented in our study. Also, the clinical manifestations and mortality pattern of SLE differ in different stages of the disease.37 Our study reflects the infection pattern in the early stages of the disease process.
In this study, the occurrence of infection was expressed as a survival function. The occurrence of major infections appeared to follow a ‘decrescendo’ pattern, with the majority of events occurring early in the course of the disease. This may reflect their susceptibility to infection as a result of the inherent immune dysregulation in SLE patients.38 These patients should be followed-up to see whether the rate and pattern of infections change as a result of cumulative increase in end-organ damage and immunosuppressive therapies.
Corticosteroid and other immunosuppressive therapies did not appear to confer excessive infective risk in our cohort. Many patients were steroid-naive (33%), and 37.0% received an average dose of ⩽10 mg/day prednisolone in the month preceding their first major infection. Only 7.4% received either azathioprine or cyclophosphamide prior to infection. Corticosteroids and immunosuppressive agents have a dual effect on the immune system in SLE.1 Corticosteroids can inhibit migration and accumulation of leukocytes; bactericidal activity, Fc receptor binding and other functions of monocytes and macrophages are often impaired. Glucocorticoids induce lymphopenia by redistributing cells to various tissue compartments, and T lymphocytes are selectively depleted. Expression of the adhesion molecule L-selectin is downregulated, thereby inhibiting lymphocyte migration to lymph nodes. Corticosteroids also impair lymphocyte proliferation and cell-mediated cytotoxicity.39 However, by suppressing abnormal cells, corticosteroids may improve the function of other aspects of the immune system. For example, abnormal neutrophil migration in untreated patients may be normalized in steroid-treated SLE patients,40 and cell-mediated immunity may improve after prednisolone therapy.41 These may contribute to the inconsistent findings regarding the role of corticosteroids as a risk factor for infections in SLE.
Active lupus is recognized as a risk factor for infection,9–11 but few studies have documented disease activity using validated instruments.16,42 In this study, we used the SLEDAI, a widely accepted instrument.21 Mean SLEDAI at the time of diagnosis of our patients was 8.2 ± 5.0 (median 7, range 0–24), which is similar to those reported in other case series of hospitalized SLE patients.16,42 There was a trend for increased risk of infection in patients with more active disease (SLEDAI >7), (p = 0.058 by log rank test), but this was not significant on multivariate analysis.
Previous studies looking at leukopenia as a predictor of infection in SLE have yielded negative results.9,10 However, total white cell count is an imprecise representation of leukocyte components, and lymphopenia can occur with a ‘normal’ total white cell count. In our patients, the prevalence of leukopenia (<4 × 109/l) was only 27%, whereas lymphopenia (<1.5 × 109/l) was present in 62% of patients at presentation (Table 1). Moreover, depletion of different cellular components may predispose to infections with different pathogens.43,44 We therefore advocate measuring differential leukocyte counts for patients with SLE.
To our knowledge this is the first study to document lymphopenia as a risk factor for infections in SLE patients. As illustrated in Figure 2, the risk of developing a major infection over the six-year period was increased by approximately 5-fold for patients with lymphocyte counts ⩽1.0 × 109/l at presentation. Since lymphocyte count in SLE patients may vary with time and disease activity, and could be modified by treatment,24,25 the lymphocyte count may fluctuate during the course of disease. In this cohort, the prevalence of lymphopenia ⩽1.0 × 109/l had increased from 49% to 66% by the end of the follow-up period. We repeated our analyses, using data censored at 12 months from diagnosis. As shown in Figure 3, the presence of lymphopenia ⩽1.0 × 109/l remained strongly predictive for the occurrence of major infection.
There is surprisingly little literature on lymphopenia in SLE, despite its common occurrence in this disorder.24,25,45,46 Lymphopenia is well known to predispose to infections, especially in patients infected with human immunodeficiency virus (HIV)44 and those with idiopathic CD4+ T-lymphocytopenia.47 Patients with a CD4 count of <0.2 × 109/l have a dramatic increase in the incidence of opportunistic infections such as Pneumocystis carinii pneumonia44 and tuberculosis.48 The mechanism of lymphopenia in SLE is not clear. Lymphopenia commonly develops in SLE patients during active disease24,45 and is strongly associated with cold-reactive, complement-fixing, and presumably cytotoxic anti-lymphocyte antibodies.49 Another potential mechanism of lymphopenia is increased apoptosis, as reflected by increased expression of Fas antigen on T cells.50
Apart from the aforementioned clinical risk factors, there are other potential contributing factors to the risk of infections in SLE patients that have not been thoroughly investigated. For instance, the integument and mucosal barriers may break down as a result of vasculitis. SLE patients may have a low serum level of mannose-binding lectin (MBL),51 which is an important protein in the opsonization and phagocytosis of microbes, leading to complement activation and deposition.17,52 In addition, multiple defects in the macrophage/monocyte system, ‘natural killer’ cells, as well as T and B cells, are common.38 Autosplenectomy and functional hyposplenia may also confer infection risk.53 These factors may contribute to the inconsistent findings reported in previous studies of risk factors for infections in SLE.
Our study is limited by its retrospective nature and relatively small sample size. We included only newly diagnosed patients, and the findings may not be applicable to other patient groups. Only the first major infection was studied, and subsequent infections were not analysed. As mentioned previously, parameters such as the disease activity, lymphocyte count, cellular functions and concomitant immunosuppressive therapies are changing dynamically throughout the disease course. As this was a retrospective study, lymphocyte count during follow-up was not measured by our standard protocol, so the effect of persistent lymphopenia on the risk of infection could not be ascertained. In future studies, patients should be followed prospectively from an early stage and monitored serially to correlate the occurrence of various types of infections with different variables.
In our SLE patients, infections were common and lymphopenia was an important risk factor for infection. Further studies on lymphocyte subsets should be performed to delineate the quantitative and qualitative immune defects associated with increased risk of infections in SLE patients.
References
Ginzler E, Berg A. Mortality in systemic lupus erythematous.
Harris EN, Williams E, Shah DJ, De Ceulaer K. Mortality of Jamaican patients with systemic lupus erythematosus.
Mok CC, Lee KW, Ho CT, Lau CS, Wong RW. A prospective study of survival and prognostic indicators of systemic lupus erythematosus in a Southern Chinese population.
Staples PJ, Gerding DN, Decker JL, Gordon RS. Incidence of infection in systemic lupus erythematosus.
Tsokos GC. Overview of cellular immune function in systemic lupus erythematosus. In: Lahita RG, ed.
Fessler BJ. Infectious diseases in systemic lupus erythematosus: risk factors, management and prophylaxis.
Ginzler E, Diamond H, Kaplan D, Weiner M, Schlesinger M, Seleznick M. Computer analysis of factors influencing frequency of infection in systemic lupus erythematosus.
De Luis A, Pigrau C, Pahissa, Fernandez F, Martinez-Vazquez JM. Infections in 96 cases of systemic lupus erythematosus.
Paton NI, Cheong IK, Kong NC, Segasothy M. Risk factors for infection in Malaysian patients with systemic lupus erythematosus.
Yuhara T, Takemura H, Akama T, Suzuki H, Yamane K, Kashiwagi H. Predicting infection in hospitalized patients with systemic lupus erythematosus.
Gomez J, Palazon D, Ortega G, Lucas E, Bru M, Campillo M, et al. Infections and systemic lupus erythematosus. Analysis of risk factors and prognosis. A prospective study (1979–1988).
Massardo L, Martinez ME, Baro M, Figueroa F, Rivero S, Jacobelli S. Infections in systemic lupus erythematosus.
Gladman DD, Hussain F, Ibanez D, Urowitz MB. The nature and outcome of infection in systemic lupus erythematosus.
Watanabe DK, Duffy CM, Gladman DD. Infection and disease activity in systemic lupus erythematosus: a review of hospitalised patients.
Garred P, Madsen HO, Halberg P, Petersen J, Kronborg G, Svejgaard A, et al. Mannose-binding lectin polymorphisms and susceptibility to infection in systemic lupus erythematosus.
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus.
ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes.
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE.
Hawker G, Gabriel S, Bombardier C, Goldsmith C, Caron D, Gladman D. A reliability study of SLEDAI: a disease activity index for systemic lupus erythematosus.
FitzGerald JD, Grossman JM. Validity and reliability of retrospective assessment of disease activity and flare in observational cohorts of lupus patients.
Barr SG, Zonana-Nacach A, Magder LS, Petri M. Patterns of disease activity in systemic lupus erythematosus.
Rivero SJ, Diaz-Jouanen E, Alarcon-Segovia D. Lymphopenia in systemic lupus erythematosus.
Nossent JC, Swaak AJG. Prevalence and significance of haematological abnormalities in patients with systemic lupus erythematosus.
Lee P, Urowitz MB, Bookman AA, Koehler BE, Smythe HA, Gordon DA, et al. Systemic lupus erythematosus. A review of 110 cases with reference to nephritis, the nervous system, infections, aseptic necrosis and prognosis.
Johnsson H, Nived O, Sturfelt G. Outcome in systemic lupus erythematosus: a prospective study of patients from a defined population.
Petri M, Genovese M. Incidence of and risk factors for hospitalisations in systemic lupus erythematosus: a prospective study of the Hopkins Lupus cohort.
Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 5-year period. A multicenter prospective study of 1,000 patients. European Working Party on Systemic Lupus Erythematosus.
Kraus A, Cabral AR, Sifuentes-Osorrnio JS, Alargcon-Segovia D. Listeriosis in patients with connective tissue diseases.
Victorio-Navarra ST, Dy EE, Arroyo CG, Torralba T. Tuberculosis among Filipino patients with systemic lupus erythematosus.
Kahl LE. Herpes zoster infections in systemic lupus erythematosus: Risk factors and outcome.
Hellmann DB, Petri M, Whiting-O’Keefe Q. Fatal infections in systemic lupus erythematosus: role of opportunistic organisms.
Zimmerman B, Spiegel M, Lally EV. Cryptococcal meningitis in systemic lupus erythematosus.
Mok CC, Lau CS, Poon SP. Primary nocardial meningitis in systemic lupus erythematosus.
Rubin LA, Urowitz MB, Gladman DD. Mortality in systemic lupus erythematosus; the bimodal pattern revisited.
Iliopoulos AG, Tsokos GC. Immunopathogenesis and spectrum of infections in systemic lupus erythematosus.
Quismorio FP. Systemic corticosteroid therapy in systemic lupus erythematosus. In: Wallace DJ, Hahn BH, eds.
Al-Hadithy H, Isenberg DA, Addison IE, Goldstone AH, Snaith ML. Neutrophil function in systemic lupus erythematosus and other collagen diseases.
Rosenthal CJ, Franklin EC. Depression of cellular-mediated immunity in systemic lupus erythematosus.
Janwityanuchit S, Totemchokchyakarn K, Krachagwongchair K, Vatanasuk M. Infection in systemic lupus erythemaotus.
Stansell JD, Osmond DH, Charlebois E, La Vange L, Wallace JM, Alexander BV, et al. Predictors of pneumocystis carinii pneumonia in HIV-infected persons.
Delbarre F, Pompidou A, Hahan A, Brouihlet H, LeGoA, Amor B. Study of lymphocytes during systemic lupus erythematosus.
Keeling DM, Isenbery DA. Haematological manifestations of systemic lupus erythematosus.
Spira TJ, Jones BM, Nicholson JK, Lai RB, Rowe T, Mawle AC, et al. Idiopathic CD4+ T-lymphocytopenia – an analysis of five patients with unexplained opportunistic infections.
Moreno S, Baraia-Etxaburu J, Bouza E, Parras F, Perez-Tascon M, Miralles P, et al. Risk for developing tuberculosis among anergic patients infected with HIV.
Winfield JB, Winchester RJ, Kunkel HG. Association of cold-reactive anti-lymphocyte antibodies with lymphopenia in systemic lupus erythematosus.
Amasaki Y, Kobayashi S, Takeda T, Ogura N, Jodo S, Nakabayashi T, et al. Up-regulated expression of Fas Ag (CD 95) by peripheral naïve and memory cells subsets in patients with systemic lupus erythematosus: a possible mechanism for lymphopenia.
Lau YL, Lau CS, Chan SY, Karlberg J, Turner MW. Mannose-binding protein in Chinese patients with systemic lupus erythematosus.
Holers VM. Complement deficiency deficiency states, disease susceptibility, and infection risk in systemic lupus erythematosus.
Author notes
From the 1Division of Rheumatology, Department of Medicine & Geriatrics, United Christian Hospital, and 2Center of Infection, The University of Hong Kong, Hong Kong Special Administrative Region, China