-
PDF
- Split View
-
Views
-
Cite
Cite
Rekha Lopez, Julie E. Davidson, Matthew D. Beeby, Peter J. Egger, David A. Isenberg, Lupus disease activity and the risk of subsequent organ damage and mortality in a large lupus cohort, Rheumatology, Volume 51, Issue 3, March 2012, Pages 491–498, https://doi.org/10.1093/rheumatology/ker368
Close - Share Icon Share
Abstract
Objectives. To estimate the effect of SLE disease activity, observed over a 12-month period, on the risk of irreversible organ damage and mortality, adjusted for potential confounding factors.
Methods. Patients were enrolled into a prospective cohort study and followed up from 1991. This study retrospectively analyses the data captured in the prospective cohort study. The study population consisted of 350 patients with SLE (meeting four or more of the revised ACR criteria) enrolled at University College Hospital, London lupus clinic. Disease activity was assessed during the observation year using the classic BILAG system and a mean total BILAG score was calculated for that time period. Organ damage outcomes, assessed over a subsequent follow-up period, were based on SLICC/ACR damage index scores and included new damage overall and by specific organ systems (renal, CNS or cardiovascular/musculoskeletal/pulmonary systems) or reaching a serious level of damage (SDI ≥ 3). Adjusted hazard ratios (HRs) for the association between disease activity and subsequent organ damage or mortality were calculated using Cox proportional hazards regression.
Results. Disease activity as measured by mean total BILAG score was associated with mortality (HR = 1.15, P = 0.008), new organ damage (HR = 1.08, P = 0.009) and CV/pulmonary or musculoskeletal damage (HR = 1.11, P = 0.007) after adjustment for age, sex, ethnicity, SLE duration, steroid exposure level, NSAID, anti-malarial or immunosuppressant use, renal activity and complement C3 or anti-dsDNA levels. Of these adjustment factors, age, renal activity, immunosuppressant use and pre-existing organ damage were additional independent predictors.
Conclusions. Disease activity as measured by global BILAG score during a 12-month observation period predicts the risk of subsequent organ damage and mortality after adjustment for key covariates.
Introduction
The survival and outcome of patients with SLE have improved dramatically over the past 50 years with improvements seen in both mortality and morbidity rates [1–3]. However, as SLE is a chronic autoimmune condition that affects a wide range of organs and systems, including the skin, musculoskeletal system, kidneys and CNS, prolonged survival rates mean an increased exposure to drugs to control disease activity for a longer period. Both disease activity and drug therapy predispose to permanent damage.
The natural history of the disease varies from relatively benign to rapidly progressive and even fatal. SLE fluctuates throughout its course and its features vary greatly between affected individuals. Disease activity can be measured using the BILAG index. As originally established, it records activity in eight separate organs or systems at each clinic visit. The index is based upon the principle of the physician's intention to treat with disease-modifying therapy [4]. The BILAG index has been shown to be a reliable and valid method of measuring disease activity and correlates highly with other commonly used indices of disease activity, such as the SLEDAI and the SLAM [5–7]. Disease flares scored as A indicate a severe disease activity defined as being likely to require >20 mg prednisone, whereas a B score indicates moderate activity, scores of C indicate mild disease and D and E indicate inactivity in that particular organ system, with D indicating previous organ involvement and E indicating no previous activity in that organ system. Organ damage is measured using the SLICC/ACR damage index (SDI) score [8].
Even though it has been generally accepted that high levels of disease activity over time increase the risk of subsequent organ damage, few studies have attempted to quantify this association in detail and report specific estimates of relative risk. A previous study in University College Hospital (UCH) reviewed a cohort of 141 patients with SLE, and found that a higher mean BILAG score was associated with a higher risk of organ damage or mortality at Year 5 [4]. Another study of 158 Norwegian patients calculated disease activity over time using the weighted average SLEDAI score and estimated an increased hazard ratio (HR) for the risk of serious organ damage (SDI score >3) compared with patients with lower weighted average SLEDAI score [9]. Disease activity has been found to be associated with increased risk of damage in inception cohorts of SLE patients free of damage at baseline [3, 10]. The aim of this study of a large and well-characterized cohort of lupus patients was to estimate the effect of SLE disease activity, including average activity over a 12-month period as measured using the total BILAG score, on the risk of irreversible organ damage and mortality during ∼10 years of follow-up, with adjustment for potential confounding factors. Subgroups at high risk of progression to organ damage or death were also sought.
Methods
Study population
Patients were enrolled into a prospective cohort study. Data were collected as part of routine clinical assessments. This current study retrospectively analyses the data captured in the prospective cohort study. Eligible patients were selected from a multiracial cohort of patients followed up after 1991 at the specialist SLE outpatient clinic at UCH and consecutively recruited into the study cohort. Each patient fulfilled the revised ACR criteria for the classification of disease [11]. Study information was recorded from clinic and hospital visits and patient data were de-identified. To be eligible for the current study, patients were required to have made at least one visit during each of (i) the 12-month period after clinic enrolment, (ii) the subsequent 12-month period (the observation year) and (iii) at any time following the observation year. This restriction ensured that patients were able to contribute a minimum amount of information required for the study analyses.
Description of predictive factors
SLE disease activity and other potential predictors of organ damage and mortality were assessed during a 12-month observation period and the study outcomes were observed over the time period that follows these 12 months. A 12-month observation period was selected to allow for better characterization of patients’ disease activity and medication use than would have been possible had predictors been observed at a single clinic visit only. The 12-month observation period was chosen to begin 1 year after a patient's first clinic visit (cohort entry date) to allow for stabilization of care. Disease activity was assessed using the classic BILAG system [5]. A total BILAG score for each study visit was calculated by assigning the following numerical values to each BILAG score (A = 12, B = 5, C = 1, D = 0, E = 0) [12] and summing the scores across organs and systems. The mean score across study visits was then calculated using a methodology developed previously for the SLEDAI score [13], that takes into account the length of time spent at a given activity level. Renal activity was recorded using the BILAG score during the observation year. Steroid exposure was estimated as the average daily dose of prednisone equivalent (milligrams) in the observation year. Anti-dsDNA (measured by ELISA, Shield Diagnostics, Dundee, UK) and complement C3 levels (measured by laser nephelometer) were assessed at each clinic visit and are reported as a mean across visits during the observation year. The use of HCQ, NSAIDs and immunosuppressant drugs were defined as ever used during the observation year (yes/no). Organ damage before the start of follow-up for outcomes was ascertained through the SDI score accrued in the clinical history until then.
Description of study outcomes
The study outcomes are described in Table 1. Organ damage was assessed with the SDI during the active follow-up period [8] and SDI scores were assigned retrospectively following chart review for organ damage. Various organ damage outcomes were defined to reflect the heterogeneity of SLE natural history observed in the clinic and to capture potentially diverse organ damage aetiologies. Patients who had experienced the outcome of interest before the start of follow-up were excluded from the analysis population for that outcome.
Description of study outcomes and analysis populations
| Study outcome . | Analysis population . |
|---|---|
| New damage (SDI Δ ≥ 1): accrual of new SDI score in any organ system | Total study cohort (n = 350) |
| New renal damage (renal SDI Δ ≥ 1): accrual of new renal SDI score | Patients with a renal SDI score of< 3 at start of follow-up (n = 347) |
| New CNS damage (CNS SDI Δ ≥ 1): accrual of new neuropsychiatric SDI score | Patients with a neuropsychiatric SDI score of <5 at start of follow-up (n = 350) |
| New cardiovascular, pulmonary or musculoskeletal damage (CV, pulmonary or MSC SDI Δ ≥ 1): accrual of new SDI score in any of these systems | Patients with a combined SDI score of <17 across CV, pulmonary or MSC domains at start of follow-up (n = 350) |
| Severe damage: accrual of a total SDI score of ≥3 | Patients with a total SDI score of <3 at start of follow-up (n = 339) |
| Mortality | Total study cohort (n = 350) |
| Study outcome . | Analysis population . |
|---|---|
| New damage (SDI Δ ≥ 1): accrual of new SDI score in any organ system | Total study cohort (n = 350) |
| New renal damage (renal SDI Δ ≥ 1): accrual of new renal SDI score | Patients with a renal SDI score of< 3 at start of follow-up (n = 347) |
| New CNS damage (CNS SDI Δ ≥ 1): accrual of new neuropsychiatric SDI score | Patients with a neuropsychiatric SDI score of <5 at start of follow-up (n = 350) |
| New cardiovascular, pulmonary or musculoskeletal damage (CV, pulmonary or MSC SDI Δ ≥ 1): accrual of new SDI score in any of these systems | Patients with a combined SDI score of <17 across CV, pulmonary or MSC domains at start of follow-up (n = 350) |
| Severe damage: accrual of a total SDI score of ≥3 | Patients with a total SDI score of <3 at start of follow-up (n = 339) |
| Mortality | Total study cohort (n = 350) |
Description of study outcomes and analysis populations
| Study outcome . | Analysis population . |
|---|---|
| New damage (SDI Δ ≥ 1): accrual of new SDI score in any organ system | Total study cohort (n = 350) |
| New renal damage (renal SDI Δ ≥ 1): accrual of new renal SDI score | Patients with a renal SDI score of< 3 at start of follow-up (n = 347) |
| New CNS damage (CNS SDI Δ ≥ 1): accrual of new neuropsychiatric SDI score | Patients with a neuropsychiatric SDI score of <5 at start of follow-up (n = 350) |
| New cardiovascular, pulmonary or musculoskeletal damage (CV, pulmonary or MSC SDI Δ ≥ 1): accrual of new SDI score in any of these systems | Patients with a combined SDI score of <17 across CV, pulmonary or MSC domains at start of follow-up (n = 350) |
| Severe damage: accrual of a total SDI score of ≥3 | Patients with a total SDI score of <3 at start of follow-up (n = 339) |
| Mortality | Total study cohort (n = 350) |
| Study outcome . | Analysis population . |
|---|---|
| New damage (SDI Δ ≥ 1): accrual of new SDI score in any organ system | Total study cohort (n = 350) |
| New renal damage (renal SDI Δ ≥ 1): accrual of new renal SDI score | Patients with a renal SDI score of< 3 at start of follow-up (n = 347) |
| New CNS damage (CNS SDI Δ ≥ 1): accrual of new neuropsychiatric SDI score | Patients with a neuropsychiatric SDI score of <5 at start of follow-up (n = 350) |
| New cardiovascular, pulmonary or musculoskeletal damage (CV, pulmonary or MSC SDI Δ ≥ 1): accrual of new SDI score in any of these systems | Patients with a combined SDI score of <17 across CV, pulmonary or MSC domains at start of follow-up (n = 350) |
| Severe damage: accrual of a total SDI score of ≥3 | Patients with a total SDI score of <3 at start of follow-up (n = 339) |
| Mortality | Total study cohort (n = 350) |
Statistical analyses
Incidence rates were calculated for each study outcome and are reported per 1000 patient-years of follow-up. Cox proportional hazards regression models were created to assess the effect of disease activity on subsequent risk of each study outcome with adjustment for covariates [14]. All potential predictive factors were assessed for association with each outcome in univariate models and in a multiple regression model. The proportionality of hazards assumption for Cox proportional hazards modelling was assessed through review of Kaplan–Meier survival curves. All analyses were performed using SAS software, version 9.1 for Windows (SAS, Cary, NC, USA).
Results
The characteristics of the study population are shown in Table 2. During the 12-month observation period, patients made a median of 4 clinic visits (range 1–12). The median follow-up time per patient was 9 years. The incidence of each study outcome during the follow-up period is presented in Table 3. By the end of follow-up, ∼40% of patients experienced new organ damage, 10% had accrued a serious level of organ damage (SDI score ≥3) and 10% had died.
Characteristics of study population (n = 350)
| Characteristic . | Median (range) unless otherwise stated . |
|---|---|
| Age at start of follow-up, years | 36 (18–78) |
| Disease duration at start of follow-up, years | 6 (0–34) |
| Decade of SLE diagnosis, n (%) | |
| 1960–69 | 3 (0.9) |
| 1970–79 | 25 (7.1) |
| 1980–89 | 99 (28.3) |
| 1990–99 | 141 (40.3) |
| 2000–10 | 82 (23.4) |
| Gender: female, n (%) | 322 (92.0) |
| Ethnicity, n (%) | |
| Caucasian | 222 (63.4) |
| Afro-Caribbean | 60 (17.1) |
| Asian (Indian) | 38 (10.9) |
| Asian (Other) | 20 (5.7) |
| Other | 10 (2.9) |
| ANA positive (yes), n (%)a | 344 (98.3) |
| SDI score at start of follow-up, n (%) | |
| 0 | 255 (72.9) |
| 1 | 62 (17.7) |
| >2 | 33 (9.4) |
| During observation year | |
| Adjusted mean total BILAG score | 4.3 (0–17.5) |
| Average daily dose of steroid (prednisone or prednisone equivalent), mg/day | 6.6 (0.3–30.0) |
| High dose of steroid (oral steroid dose >20 mg or intramuscular or pulse steroid therapy) (yes), n (%) | 37 (10.6) |
| Mean Anti-dsDNA, mean (s.d.),b IU/ml | 137.6 (282.4) |
| Anti-dsDNA >50 IU/ml on at least once clinic visit (yes), n (%) | 212 (60.6) |
| Mean Complement level C3, mean (s.d.), mg/dlc | 0.96 (0.33) |
| Complement level CS ≤0.9 mg/dl on at least once clinic visit (yes), n (%) | 200 (57.1) |
| NSAID use (yes), n (%) | 116 (33.1) |
| HCQ use (yes), n (%) | 192 (54.9) |
| Immunosuppressant/immune-modulator use (yes), n (%) | 158 (45.1) |
| Characteristic . | Median (range) unless otherwise stated . |
|---|---|
| Age at start of follow-up, years | 36 (18–78) |
| Disease duration at start of follow-up, years | 6 (0–34) |
| Decade of SLE diagnosis, n (%) | |
| 1960–69 | 3 (0.9) |
| 1970–79 | 25 (7.1) |
| 1980–89 | 99 (28.3) |
| 1990–99 | 141 (40.3) |
| 2000–10 | 82 (23.4) |
| Gender: female, n (%) | 322 (92.0) |
| Ethnicity, n (%) | |
| Caucasian | 222 (63.4) |
| Afro-Caribbean | 60 (17.1) |
| Asian (Indian) | 38 (10.9) |
| Asian (Other) | 20 (5.7) |
| Other | 10 (2.9) |
| ANA positive (yes), n (%)a | 344 (98.3) |
| SDI score at start of follow-up, n (%) | |
| 0 | 255 (72.9) |
| 1 | 62 (17.7) |
| >2 | 33 (9.4) |
| During observation year | |
| Adjusted mean total BILAG score | 4.3 (0–17.5) |
| Average daily dose of steroid (prednisone or prednisone equivalent), mg/day | 6.6 (0.3–30.0) |
| High dose of steroid (oral steroid dose >20 mg or intramuscular or pulse steroid therapy) (yes), n (%) | 37 (10.6) |
| Mean Anti-dsDNA, mean (s.d.),b IU/ml | 137.6 (282.4) |
| Anti-dsDNA >50 IU/ml on at least once clinic visit (yes), n (%) | 212 (60.6) |
| Mean Complement level C3, mean (s.d.), mg/dlc | 0.96 (0.33) |
| Complement level CS ≤0.9 mg/dl on at least once clinic visit (yes), n (%) | 200 (57.1) |
| NSAID use (yes), n (%) | 116 (33.1) |
| HCQ use (yes), n (%) | 192 (54.9) |
| Immunosuppressant/immune-modulator use (yes), n (%) | 158 (45.1) |
aANA-positive titre ≥1 : 80. bAnti-dsDNA (ELISA) normal ≤ 50 IU/ml. cComplement C3 normal range = 0.9–1.8 mg/dl.
Characteristics of study population (n = 350)
| Characteristic . | Median (range) unless otherwise stated . |
|---|---|
| Age at start of follow-up, years | 36 (18–78) |
| Disease duration at start of follow-up, years | 6 (0–34) |
| Decade of SLE diagnosis, n (%) | |
| 1960–69 | 3 (0.9) |
| 1970–79 | 25 (7.1) |
| 1980–89 | 99 (28.3) |
| 1990–99 | 141 (40.3) |
| 2000–10 | 82 (23.4) |
| Gender: female, n (%) | 322 (92.0) |
| Ethnicity, n (%) | |
| Caucasian | 222 (63.4) |
| Afro-Caribbean | 60 (17.1) |
| Asian (Indian) | 38 (10.9) |
| Asian (Other) | 20 (5.7) |
| Other | 10 (2.9) |
| ANA positive (yes), n (%)a | 344 (98.3) |
| SDI score at start of follow-up, n (%) | |
| 0 | 255 (72.9) |
| 1 | 62 (17.7) |
| >2 | 33 (9.4) |
| During observation year | |
| Adjusted mean total BILAG score | 4.3 (0–17.5) |
| Average daily dose of steroid (prednisone or prednisone equivalent), mg/day | 6.6 (0.3–30.0) |
| High dose of steroid (oral steroid dose >20 mg or intramuscular or pulse steroid therapy) (yes), n (%) | 37 (10.6) |
| Mean Anti-dsDNA, mean (s.d.),b IU/ml | 137.6 (282.4) |
| Anti-dsDNA >50 IU/ml on at least once clinic visit (yes), n (%) | 212 (60.6) |
| Mean Complement level C3, mean (s.d.), mg/dlc | 0.96 (0.33) |
| Complement level CS ≤0.9 mg/dl on at least once clinic visit (yes), n (%) | 200 (57.1) |
| NSAID use (yes), n (%) | 116 (33.1) |
| HCQ use (yes), n (%) | 192 (54.9) |
| Immunosuppressant/immune-modulator use (yes), n (%) | 158 (45.1) |
| Characteristic . | Median (range) unless otherwise stated . |
|---|---|
| Age at start of follow-up, years | 36 (18–78) |
| Disease duration at start of follow-up, years | 6 (0–34) |
| Decade of SLE diagnosis, n (%) | |
| 1960–69 | 3 (0.9) |
| 1970–79 | 25 (7.1) |
| 1980–89 | 99 (28.3) |
| 1990–99 | 141 (40.3) |
| 2000–10 | 82 (23.4) |
| Gender: female, n (%) | 322 (92.0) |
| Ethnicity, n (%) | |
| Caucasian | 222 (63.4) |
| Afro-Caribbean | 60 (17.1) |
| Asian (Indian) | 38 (10.9) |
| Asian (Other) | 20 (5.7) |
| Other | 10 (2.9) |
| ANA positive (yes), n (%)a | 344 (98.3) |
| SDI score at start of follow-up, n (%) | |
| 0 | 255 (72.9) |
| 1 | 62 (17.7) |
| >2 | 33 (9.4) |
| During observation year | |
| Adjusted mean total BILAG score | 4.3 (0–17.5) |
| Average daily dose of steroid (prednisone or prednisone equivalent), mg/day | 6.6 (0.3–30.0) |
| High dose of steroid (oral steroid dose >20 mg or intramuscular or pulse steroid therapy) (yes), n (%) | 37 (10.6) |
| Mean Anti-dsDNA, mean (s.d.),b IU/ml | 137.6 (282.4) |
| Anti-dsDNA >50 IU/ml on at least once clinic visit (yes), n (%) | 212 (60.6) |
| Mean Complement level C3, mean (s.d.), mg/dlc | 0.96 (0.33) |
| Complement level CS ≤0.9 mg/dl on at least once clinic visit (yes), n (%) | 200 (57.1) |
| NSAID use (yes), n (%) | 116 (33.1) |
| HCQ use (yes), n (%) | 192 (54.9) |
| Immunosuppressant/immune-modulator use (yes), n (%) | 158 (45.1) |
aANA-positive titre ≥1 : 80. bAnti-dsDNA (ELISA) normal ≤ 50 IU/ml. cComplement C3 normal range = 0.9–1.8 mg/dl.
Incidence of study outcomes during follow-up
| Outcome . | Size of analysis cohort (n) . | Outcome during follow- up (n) . | Incidence per 1000 person- years . |
|---|---|---|---|
| SDI Δ ≥1 | 350 | 109 | 39.6 |
| Renal SDI Δ ≥1 | 347 | 16 | 4.8 |
| CNS SDI Δ ≥1 | 350 | 20 | 6.1 |
| CV, pulmonary or MSC SDI Δ ≥1 | 350 | 58 | 18.7 |
| Total SDI score of ≥3 | 339 | 33 | 10.4 |
| Mortality | 350 | 34 | 9.9 |
| Outcome . | Size of analysis cohort (n) . | Outcome during follow- up (n) . | Incidence per 1000 person- years . |
|---|---|---|---|
| SDI Δ ≥1 | 350 | 109 | 39.6 |
| Renal SDI Δ ≥1 | 347 | 16 | 4.8 |
| CNS SDI Δ ≥1 | 350 | 20 | 6.1 |
| CV, pulmonary or MSC SDI Δ ≥1 | 350 | 58 | 18.7 |
| Total SDI score of ≥3 | 339 | 33 | 10.4 |
| Mortality | 350 | 34 | 9.9 |
Incidence of study outcomes during follow-up
| Outcome . | Size of analysis cohort (n) . | Outcome during follow- up (n) . | Incidence per 1000 person- years . |
|---|---|---|---|
| SDI Δ ≥1 | 350 | 109 | 39.6 |
| Renal SDI Δ ≥1 | 347 | 16 | 4.8 |
| CNS SDI Δ ≥1 | 350 | 20 | 6.1 |
| CV, pulmonary or MSC SDI Δ ≥1 | 350 | 58 | 18.7 |
| Total SDI score of ≥3 | 339 | 33 | 10.4 |
| Mortality | 350 | 34 | 9.9 |
| Outcome . | Size of analysis cohort (n) . | Outcome during follow- up (n) . | Incidence per 1000 person- years . |
|---|---|---|---|
| SDI Δ ≥1 | 350 | 109 | 39.6 |
| Renal SDI Δ ≥1 | 347 | 16 | 4.8 |
| CNS SDI Δ ≥1 | 350 | 20 | 6.1 |
| CV, pulmonary or MSC SDI Δ ≥1 | 350 | 58 | 18.7 |
| Total SDI score of ≥3 | 339 | 33 | 10.4 |
| Mortality | 350 | 34 | 9.9 |
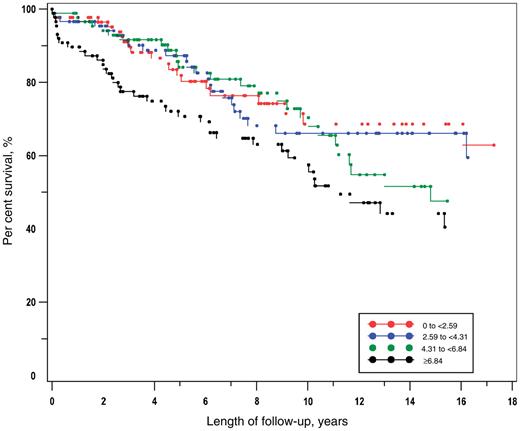
The Kaplan–Meier plots for quartiles of the adjusted mean total BILAG score were reviewed. Inspection of the different categories suggested a trend in the effect and it was judged reasonable to use the adjusted mean total BILAG score as a continuous variable in the statistical model. The Kaplan–Meier plot of time to any new organ damage (SDI Δ ≥ 1) by quartile of adjusted mean total BILAG score is presented in Fig. 1. The risk of organ damage differed across the quartiles (log-rank chi-square 8.6, P = 0.036). The plot shows that those in the highest quartile of adjusted mean total BILAG score were at an elevated risk of the outcome from the start of follow-up compared with those in the lower quartiles, while there was no clear trend between the lower quartiles. The results of the Cox proportional hazards modelling for each outcome, mutually adjusted for all other factors in the model, are presented in Table 4.
Kaplan–Meier analysis of time to SDI Δ ≥ 1 by quartile of adjusted mean total BILAG score.
Final model results from multiple Cox proportional hazards regression modelling
| Predictor . | Mortality (n = 350) . | SDI Δ ≥ 1 (n = 350) . | Renal SDI Δ ≥ 1 (n = 347) . | CNS SDI Δ ≥ 1 (n = 350) . | CV, pulmonary, or MSC SDI Δ ≥ 1 (n = 350) . | SDI ≥ 3 (n = 339) . |
|---|---|---|---|---|---|---|
| HR (p) | HR (p) | HR (p) | HR (p) | HR (p) | HR (p) | |
| Adjusted mean total BILAG | 1.15 (0.008) | 1.08 (0.009) | 1.03 (0.700) | 1.07 (0.326) | 1.11 (0.007) | 1.08 (0.144) |
| Age at start of follow-up, years | 1.06 (<0.001) | 1.03 (<0.001) | 1.02 (0.422) | 1.00 (0.868) | 1.05 (<0.001) | 1.01 (0.489) |
| Gender: female | 0.78 (0.696) | 0.72 (0.316) | NSa | NSa | 0.63 (0.311) | 0.50 (0.289) |
| Ethnicity | ||||||
| Caucasian | Ref | Ref | Ref | Ref | Ref | Ref |
| Afro-Caribbean | 0.58 (0.341) | 1.01 (0.981) | 1.00 (0.997) | 0.55 (0.456) | 1.58 (0.256) | 0.77 (0.619) |
| Other | 0.68 (0.457) | 0.97 (0.899) | 2.46 (0.197) | 1.10 (0.864) | 1.10 (0.797) | 0.92 (0.868) |
| Duration of disease at start of follow-up, years | 0.98 (0.512) | 1.00 (0.927) | 0.96 (0.496) | 1.00 (0.968) | 1.03 (0.204) | 1.05 (0.232) |
| Average daily steroid dose, mg/day | 1.05 (0.172) | 1.00 (0.998) | 0.95 (0.450) | 1.03 (0.530) | 1.01 (0.881) | 1.01 (0.880) |
| NSAID use (yes) | 0.64 (0.285) | 0.69 (0.097) | 0.35 (0.215) | 0.80 (0.660) | 0.79 (0.431) | 1.23 (0.615) |
| HCQ use (yes) | 0.50 (0.096) | 0.84 (0.408) | 0.53 (0.302) | 0.62 (0.335) | 1.04 (0.890) | 0.68 (0.371) |
| Immunosuppressant use (yes) | 1.29 (0.509) | 1.56 (0.044) | 1.83 (0.374) | 1.78 (0.251) | 1.81 (0.054) | 1.16 (0.723) |
| SDI at start of follow-up | 1.70 (0.001) | 1.11 (0.323) | 2.06 (0.026) | 1.20 (0.443) | 0.90 (0.579) | 5.71 (<0.001) |
| Renal activity (yes) | 0.79 (0.620) | 1.32 (0.275) | 15.84 (<0.001) | 0.37 (0.151) | 0.88 (0.737) | 2.73 (0.036) |
| Mean anti-dsDNA, IU/ml | 1.00 (0.814) | 1.00 (0.503) | 1.00 (0.271) | 1.00 (0.402) | 1.00 (0.129) | 1.00 (0.844) |
| Mean Complement level C3, mg/dl | 0.58 (0.460) | 1.00 (0.990) | 0.26 (0.137) | 1.15 (0.845) | 0.53 (0.259) | 0.70 (0.663) |
| Predictor . | Mortality (n = 350) . | SDI Δ ≥ 1 (n = 350) . | Renal SDI Δ ≥ 1 (n = 347) . | CNS SDI Δ ≥ 1 (n = 350) . | CV, pulmonary, or MSC SDI Δ ≥ 1 (n = 350) . | SDI ≥ 3 (n = 339) . |
|---|---|---|---|---|---|---|
| HR (p) | HR (p) | HR (p) | HR (p) | HR (p) | HR (p) | |
| Adjusted mean total BILAG | 1.15 (0.008) | 1.08 (0.009) | 1.03 (0.700) | 1.07 (0.326) | 1.11 (0.007) | 1.08 (0.144) |
| Age at start of follow-up, years | 1.06 (<0.001) | 1.03 (<0.001) | 1.02 (0.422) | 1.00 (0.868) | 1.05 (<0.001) | 1.01 (0.489) |
| Gender: female | 0.78 (0.696) | 0.72 (0.316) | NSa | NSa | 0.63 (0.311) | 0.50 (0.289) |
| Ethnicity | ||||||
| Caucasian | Ref | Ref | Ref | Ref | Ref | Ref |
| Afro-Caribbean | 0.58 (0.341) | 1.01 (0.981) | 1.00 (0.997) | 0.55 (0.456) | 1.58 (0.256) | 0.77 (0.619) |
| Other | 0.68 (0.457) | 0.97 (0.899) | 2.46 (0.197) | 1.10 (0.864) | 1.10 (0.797) | 0.92 (0.868) |
| Duration of disease at start of follow-up, years | 0.98 (0.512) | 1.00 (0.927) | 0.96 (0.496) | 1.00 (0.968) | 1.03 (0.204) | 1.05 (0.232) |
| Average daily steroid dose, mg/day | 1.05 (0.172) | 1.00 (0.998) | 0.95 (0.450) | 1.03 (0.530) | 1.01 (0.881) | 1.01 (0.880) |
| NSAID use (yes) | 0.64 (0.285) | 0.69 (0.097) | 0.35 (0.215) | 0.80 (0.660) | 0.79 (0.431) | 1.23 (0.615) |
| HCQ use (yes) | 0.50 (0.096) | 0.84 (0.408) | 0.53 (0.302) | 0.62 (0.335) | 1.04 (0.890) | 0.68 (0.371) |
| Immunosuppressant use (yes) | 1.29 (0.509) | 1.56 (0.044) | 1.83 (0.374) | 1.78 (0.251) | 1.81 (0.054) | 1.16 (0.723) |
| SDI at start of follow-up | 1.70 (0.001) | 1.11 (0.323) | 2.06 (0.026) | 1.20 (0.443) | 0.90 (0.579) | 5.71 (<0.001) |
| Renal activity (yes) | 0.79 (0.620) | 1.32 (0.275) | 15.84 (<0.001) | 0.37 (0.151) | 0.88 (0.737) | 2.73 (0.036) |
| Mean anti-dsDNA, IU/ml | 1.00 (0.814) | 1.00 (0.503) | 1.00 (0.271) | 1.00 (0.402) | 1.00 (0.129) | 1.00 (0.844) |
| Mean Complement level C3, mg/dl | 0.58 (0.460) | 1.00 (0.990) | 0.26 (0.137) | 1.15 (0.845) | 0.53 (0.259) | 0.70 (0.663) |
aHRs inestimable due to zero cells. Statistically significant HRs (P < 0.05) are presented in bold.
Final model results from multiple Cox proportional hazards regression modelling
| Predictor . | Mortality (n = 350) . | SDI Δ ≥ 1 (n = 350) . | Renal SDI Δ ≥ 1 (n = 347) . | CNS SDI Δ ≥ 1 (n = 350) . | CV, pulmonary, or MSC SDI Δ ≥ 1 (n = 350) . | SDI ≥ 3 (n = 339) . |
|---|---|---|---|---|---|---|
| HR (p) | HR (p) | HR (p) | HR (p) | HR (p) | HR (p) | |
| Adjusted mean total BILAG | 1.15 (0.008) | 1.08 (0.009) | 1.03 (0.700) | 1.07 (0.326) | 1.11 (0.007) | 1.08 (0.144) |
| Age at start of follow-up, years | 1.06 (<0.001) | 1.03 (<0.001) | 1.02 (0.422) | 1.00 (0.868) | 1.05 (<0.001) | 1.01 (0.489) |
| Gender: female | 0.78 (0.696) | 0.72 (0.316) | NSa | NSa | 0.63 (0.311) | 0.50 (0.289) |
| Ethnicity | ||||||
| Caucasian | Ref | Ref | Ref | Ref | Ref | Ref |
| Afro-Caribbean | 0.58 (0.341) | 1.01 (0.981) | 1.00 (0.997) | 0.55 (0.456) | 1.58 (0.256) | 0.77 (0.619) |
| Other | 0.68 (0.457) | 0.97 (0.899) | 2.46 (0.197) | 1.10 (0.864) | 1.10 (0.797) | 0.92 (0.868) |
| Duration of disease at start of follow-up, years | 0.98 (0.512) | 1.00 (0.927) | 0.96 (0.496) | 1.00 (0.968) | 1.03 (0.204) | 1.05 (0.232) |
| Average daily steroid dose, mg/day | 1.05 (0.172) | 1.00 (0.998) | 0.95 (0.450) | 1.03 (0.530) | 1.01 (0.881) | 1.01 (0.880) |
| NSAID use (yes) | 0.64 (0.285) | 0.69 (0.097) | 0.35 (0.215) | 0.80 (0.660) | 0.79 (0.431) | 1.23 (0.615) |
| HCQ use (yes) | 0.50 (0.096) | 0.84 (0.408) | 0.53 (0.302) | 0.62 (0.335) | 1.04 (0.890) | 0.68 (0.371) |
| Immunosuppressant use (yes) | 1.29 (0.509) | 1.56 (0.044) | 1.83 (0.374) | 1.78 (0.251) | 1.81 (0.054) | 1.16 (0.723) |
| SDI at start of follow-up | 1.70 (0.001) | 1.11 (0.323) | 2.06 (0.026) | 1.20 (0.443) | 0.90 (0.579) | 5.71 (<0.001) |
| Renal activity (yes) | 0.79 (0.620) | 1.32 (0.275) | 15.84 (<0.001) | 0.37 (0.151) | 0.88 (0.737) | 2.73 (0.036) |
| Mean anti-dsDNA, IU/ml | 1.00 (0.814) | 1.00 (0.503) | 1.00 (0.271) | 1.00 (0.402) | 1.00 (0.129) | 1.00 (0.844) |
| Mean Complement level C3, mg/dl | 0.58 (0.460) | 1.00 (0.990) | 0.26 (0.137) | 1.15 (0.845) | 0.53 (0.259) | 0.70 (0.663) |
| Predictor . | Mortality (n = 350) . | SDI Δ ≥ 1 (n = 350) . | Renal SDI Δ ≥ 1 (n = 347) . | CNS SDI Δ ≥ 1 (n = 350) . | CV, pulmonary, or MSC SDI Δ ≥ 1 (n = 350) . | SDI ≥ 3 (n = 339) . |
|---|---|---|---|---|---|---|
| HR (p) | HR (p) | HR (p) | HR (p) | HR (p) | HR (p) | |
| Adjusted mean total BILAG | 1.15 (0.008) | 1.08 (0.009) | 1.03 (0.700) | 1.07 (0.326) | 1.11 (0.007) | 1.08 (0.144) |
| Age at start of follow-up, years | 1.06 (<0.001) | 1.03 (<0.001) | 1.02 (0.422) | 1.00 (0.868) | 1.05 (<0.001) | 1.01 (0.489) |
| Gender: female | 0.78 (0.696) | 0.72 (0.316) | NSa | NSa | 0.63 (0.311) | 0.50 (0.289) |
| Ethnicity | ||||||
| Caucasian | Ref | Ref | Ref | Ref | Ref | Ref |
| Afro-Caribbean | 0.58 (0.341) | 1.01 (0.981) | 1.00 (0.997) | 0.55 (0.456) | 1.58 (0.256) | 0.77 (0.619) |
| Other | 0.68 (0.457) | 0.97 (0.899) | 2.46 (0.197) | 1.10 (0.864) | 1.10 (0.797) | 0.92 (0.868) |
| Duration of disease at start of follow-up, years | 0.98 (0.512) | 1.00 (0.927) | 0.96 (0.496) | 1.00 (0.968) | 1.03 (0.204) | 1.05 (0.232) |
| Average daily steroid dose, mg/day | 1.05 (0.172) | 1.00 (0.998) | 0.95 (0.450) | 1.03 (0.530) | 1.01 (0.881) | 1.01 (0.880) |
| NSAID use (yes) | 0.64 (0.285) | 0.69 (0.097) | 0.35 (0.215) | 0.80 (0.660) | 0.79 (0.431) | 1.23 (0.615) |
| HCQ use (yes) | 0.50 (0.096) | 0.84 (0.408) | 0.53 (0.302) | 0.62 (0.335) | 1.04 (0.890) | 0.68 (0.371) |
| Immunosuppressant use (yes) | 1.29 (0.509) | 1.56 (0.044) | 1.83 (0.374) | 1.78 (0.251) | 1.81 (0.054) | 1.16 (0.723) |
| SDI at start of follow-up | 1.70 (0.001) | 1.11 (0.323) | 2.06 (0.026) | 1.20 (0.443) | 0.90 (0.579) | 5.71 (<0.001) |
| Renal activity (yes) | 0.79 (0.620) | 1.32 (0.275) | 15.84 (<0.001) | 0.37 (0.151) | 0.88 (0.737) | 2.73 (0.036) |
| Mean anti-dsDNA, IU/ml | 1.00 (0.814) | 1.00 (0.503) | 1.00 (0.271) | 1.00 (0.402) | 1.00 (0.129) | 1.00 (0.844) |
| Mean Complement level C3, mg/dl | 0.58 (0.460) | 1.00 (0.990) | 0.26 (0.137) | 1.15 (0.845) | 0.53 (0.259) | 0.70 (0.663) |
aHRs inestimable due to zero cells. Statistically significant HRs (P < 0.05) are presented in bold.
Immunosuppressant/immunomodulator use was associated with an increased risk of a number of outcomes (mortality SDI Δ ≥ 1, renal SDI Δ ≥ 1 and CV, pulmonary or musculoskeletal SDI Δ ≥ 1) in the univariate analyses, but only with SDI Δ ≥ 1 after adjustment for covariates (HR = 1.56, P = 0.044). HCQ use was significantly associated with a reduced risk of mortality, SDI Δ ≥ 1 and renal SDI Δ ≥ 1 in univariate models, and although the HRs (<1) continued to suggest a protective effect, no statistically significant effects were detected after adjustment for other covariates. Steroid use (milligrams per day) was not associated with any outcome after adjustment for covariates, although univariate models had suggested an association with increased risk for renal SDI Δ ≥ 1 and mortality.
Adjusted mean total BILAG score and age were the most consistent predictors of risk across the study outcomes after adjustment for all other factors. A one point increase in adjusted mean total BILAG score was associated with a 15% increase in mortality risk (HR = 1.15, P = 0.008), an 8% increase in the risk of any new organ damage (HR = 1.08, P = 0.009) and an 11% increase in risk of CV, pulmonary or musculoskeletal damage (HR = 1.11, P = 0.007); each additional year of age increased the risk of these outcomes by 6 (P < 0.001), 3 (P < 0.001) and 5% (P < 0.001), respectively.
Existing burden of organ damage (SDI score at start of follow-up) was also a significant predictor across several outcomes (mortality, renal damage and reaching a serious damage level). Renal activity was significantly associated with an increased risk of reaching a serious level of damage (SDI ≥ 3) and was a strong predictor of renal damage (HR = 15.84, P < 0.001).
Afro-Caribbean ethnicity was significantly associated with an increased risk of renal damage relative to Caucasian ethnicity in a univariate model (HR = 3.6, P = 0.034), but this association disappeared in the adjusted model (HR = 1.00, P = 0.997). The reasons for this change were explored. We assessed the contribution of each model covariate to the association between Afro-Caribbean ethnicity and renal damage in bivariate models. This analysis suggested that renal activity, a strong predictor of renal damage, was the principal confounder of the relationship.
Discussion
One of the major challenges facing clinicians managing patients with SLE is to predict the disease course and prevent irreversible organ damage, which impacts on patient outcome. Despite improved survival rates, patients with SLE are reported to have a 4.6-fold higher standardized mortality rate compared with the general population [15]. The mortality and morbidity rates observed in the current cohort of patients help illustrate this fact.
The link between disease activity and damage has been established previously and this has been confirmed in our cohort of 350 patients. A one-point increase in adjusted mean total BILAG score, observed over a 12-month period, was associated with a 15% increased risk of mortality (corresponding to a reduced survival time) and an 8% increased risk of new organ damage. These data indicate that disease activity, even measured over a relatively short period of time, plays an important role in predicting patient outcome. This supports findings from previous studies. Gilboe et al. [16] in Norway showed that increased mean SLEDAI scores >2 years in a population of 93 Caucasian lupus patients were associated with higher risk of progression to organ damage and mortality. Higher disease activity at baseline, as measured by SLAM, was associated with a reduced time to initial damage in the LUMINA inception cohort [3] and disease activity, as measured by average annual SLEDAI, was associated with an increased risk of damage in a multi-national European inception cohort [10]. Nossent et al. [17] also demonstrated poorer prognosis among Afro-Caribbean lupus patients with higher disease activity measures. In our cohort, disease activity was measured using the BILAG system, which records flares in each organ system and is sensitive to change [18]. Increased disease flares result in increased mean BILAG scores and therefore independently predict increased mortality and damage. Other studies have used the SLEDAI score as a measure of disease activity and have shown that persistent disease activity and damage accrual (SDI) were associated with mortality [19]. Therefore, the control of disease activity in patients is thought to be important in reducing permanent organ damage and is likely to impact on the outcome of the patient.
In the present study, we found age, rather than duration of disease, to be predictive of increased mortality and new organ damage. Age was also a strong predictive factor for organ damage in the cardiovascular, pulmonary and musculoskeletal systems. It is possible that this finding may reflect the increased incidence of comorbidities associated with age. These results contrast with a previous study, based in the UCH Lupus Cohort (albeit with one-third the number of patients used for the current analysis, followed for half as long), which found that duration of disease was a risk factor for organ damage rather than age [20]. Other studies by Bujan et al. [21] and Bertoli et al. [22] have found that diagnosis after the age of 50 years was an independent risk factor for mortality. Important causes of death in patients with lupus include accelerated atherosclerosis [23], infection and malignancy [24], all of which tend to increase with advanced age.
In this study organ damage burden, measured by accumulated SDI score at the start of follow-up, was a significant factor in predicting mortality and further organ damage. The accumulation of organ damage, as evidenced by a high SDI score, has been shown to predict damage and increased mortality in previous studies [1–3, 15, 25]. Rahman et al. [25] showed that lupus patients with early onset damage (occurring within 1 year of disease onset) had a three times higher mortality rate at 10 years when compared with lupus patients with no early damage. The LUMINA study also attempted to identify factors relating to deaths occurring within the first 3 years in a group of 154 lupus patients. They noted that the 11 deceased patients had accrued damage more rapidly by the time of the last visit compared with those that survived, indicating early damage accrual is associated with greater risk of mortality [26]. A previous logistic regression analysis of data from 141 patients attending the UCH clinic showed the initial total damage score to be a significant predictor of damage 3 years later [20]. Fortin et al. [27] prospectively followed 95 patients with lupus who had a SDI at baseline for 4 years. Using a multivariate model, they showed that the SDI best predicted a deleterious outcome defined as hospitalization and death.
Renal activity was associated with an increased risk of renal damage and serious organ damage (SDI ≥ 3). This finding is not surprising, as activity in the renal system is generally regarded as suggesting serious lupus involvement. Strikingly, in this cohort there is a 16-fold increased risk of renal damage associated with renal activity. Stoll et al. [28] showed that the mean renal damage at 1 year significantly correlated with the development of end-stage renal failure (ESRF). This finding corresponds with known prognostic indicators of reduced glomerular filtration rate or proteinuria for poor renal outcome [29]. A recent study of 198 Pakistani patients showed that early renal damage occurring within 1 year of diagnosis is significantly associated with the development of ESRF and a significant predictor of mortality [30].
A previous study conducted at UCH on 127 patients with LN showed that Afro-Caribbean patients were more likely to develop LN (44 vs only 22% of Caucasian patients) and were also more likely to progress to ESRF [31]. In the present study, renal activity was the predominant predictor of renal damage, and 48% of Afro-Caribbean patients had renal activity during the observation year, as compared with only 19% of Caucasian patients. Afro-Caribbean patients may be at increased risk of progression to renal damage compared with Caucasian patients due to their disproportionate burden of renal activity.
We found an association between immunosuppressant use and risk of new organ damage after adjustment for confounding factors. While immunosuppressant therapy is indicated in those with severe disease who would be expected to be at highest risk of organ damage, our finding remained after adjustment for several indicators of severe diseases (average disease activity over the observation year, any renal activity and pre-existing organ damage). Immunosuppressants have been reported as causing damage such as increased incidence of malignancy, in particular haematological malignancies and carcinomas of the bladder [32, 33], in previous studies of autoimmune rheumatic diseases and in SLE in particular. This may account for our finding of an increased risk of new damage. Immunosuppressants, in particular CYC, are also associated with an increased risk of sustained amenorrhoea, which can contribute to premature gonadal failure [34]. Women >32 years of age who are treated for LN with i.v. CYC may be at increased incidence of sustained amenorrhoea even with short courses [35]. Park et al. [36] showed that older age, high cumulative dose of i.v. CYC and high damage index at the start of therapy were independent risk factors for premature gonadal failure and occurred in 14.7% of patients. Premature gonadal failure caused by CYC is due to its effect on causing follicular death by damaging the rapidly dividing granulosa cells, which produce oestrogen and progesterone to developing follicles [37]. Ovarian failure is a risk factor for other significant morbidities associated with an increased risk of damage, namely increased cardiovascular morbidity [38] as well as an increased risk of osteoporosis [39]. Our study is limited in that we did not look at the association of specific items of damage in relation to immunosuppressant use, cumulative dosage and length of treatment, but this would be of interest.
In our study, steroid use was not found to be associated with accumulation of new organ damage or mortality after adjustment for covariates. The mean daily dose of steroids in the observation year in our cohort was 6.6 mg. A recent study by Thamer et al. [40] showed that low doses of prednisone (∼6 mg/day) were not associated with an increased risk of organ damage; their data support our finding. Previous work in the Hopkins lupus cohort suggests that cumulative high doses of prednisone used over the course of the disease results in increased organ damage [41]. Our study is limited in that we did not assess total CS use over the entire follow-up period, only during the observation year.
Our study benefited from the relatively large size of our lupus cohort and the length of careful follow-up for organ damage and mortality outcomes. Disease activity in our study was measured by an adjusted mean total BILAG score. Although studies have used an adjusted mean SLEDAI score previously [13], to our knowledge this is the first time an adjusted mean BILAG score has been used to measure disease activity over time. Further work is needed using this application of the scoring system.
In conclusion, our results suggest that SLE disease activity, as measured over a period of 12 months, is associated with an increased risk of irreversible organ damage and mortality, after adjustment for potential confounding factors. In addition, age, pre-existing burden of organ damage, renal activity and use of immunosuppressant/immunomodulating drugs were identified as significant risk factors.

Acknowledgements
Funding: The work was supported by a grant from GlaxoSmithKline plc and Human Genome Sciences.
Disclosure statement: J.E.D. and P.J.E. are employees of GlaxoSmithKline R&D and hold shares in GlaxoSmithKline plc. M.D.B. is an employee of GlaxoSmithKline R&D. All other authors have declared no conflicts of interest.
References
- survival analysis
- immunosuppressive agents
- lung
- systemic lupus erythematosus
- discoid lupus erythematosus
- anti-inflammatory agents, non-steroidal
- cardiovascular system
- complement 3
- ethnic group
- follow-up
- prospective studies
- steroids
- time factors
- kidney
- lupus erythematosus
- mortality
- anti-dsdna antibody




Comments