The diagnostic utility and tendency of the soluble receptor for advanced glycation end products (sRAGE) in exudative pleural effusion
Introduction
Pleural effusions are common manifestations of a variety of diseases and many patients with a pleural effusion also suffer from dyspnea or chest discomfort. Although Light’s well-established criteria, and various methods of analyzing pleural fluid, are usually used to determine the cause of a pleural effusion, a diagnostic procedure is occasionally required that may be invasive technique or incur a high cost. In addition, ambiguous data can result in difficulty in determining the origin of pleural fluid (1).
The receptor for advanced glycation end products (RAGE) protein belongs to the immunoglobulin superfamily of cell surface molecules and acts as a recognition receptor for advanced glycation end products (AGEs) (2,3). RAGE is expressed at low levels in most normal tissues, but at high levels on the cell membranes of alveolar type I epithelial cells in the lungs, even under normal physiological conditions and is considered to have a homeostatic function (2,3) within the lung. Further studies suggested that the activation of RAGE plays a role in various inflammatory and infectious diseases (4-6), acute respiratory distress syndrome (ARDS), and acute lung injury (ALI) (7,8). Soluble receptor for advanced glycation end products (sRAGE) consists of the extracellular domain but lacks the trans-membrane and cytoplasmic domains of RAGE in human plasma (9). sRAGE may have an inflammatory or homeostatic role to play in lung tissue (5,10), and is studied as an effective biomarker in ARDS (8,10), ALI (8,10), lung cancer (11) and chronic obstructive pulmonary disease (12).
We hypothesized that there is a distinguishable difference in the level of sRAGE in pleural effusion for various diseases due to the high expression and important role of RAGE in lung tissue. The aim of this study was to assess the usefulness and tendency of sRAGE as a diagnostic marker for pleural effusion and serum.
Methods
Patients
We prospectively enrolled patients with undiagnosed pleural effusions who were referred to Hallym University Kangnam Sacred Heart Hospital between January 2013 and January 2015. Samples of pleural fluid were collected from consecutive patients who had undergone thoracentesis. When multiple thoracenteses were required, only the first sample was analyzed. Pleural fluid was centrifuged and the supernatant stored at −70 °C. The final diagnosis was agreed by two independent pulmonary physicians based on all available clinical information. Patients with effusions of uncertain etiology or effusions that remained undiagnosed were excluded from the analysis.
Ethical statement
This study received ethical approval (Institutional Review Board number 2012-11-98) from the Research Ethics Committee of Hallym University Kangnam Sacred Heart Hospital, and written informed consent was obtained from all patients.
Diagnostic criteria
Exudative pleural effusion was determined if one of the following Light’s criteria was fulfilled: an effusion protein/serum protein ratio greater than 0.5, an effusion lactate dehydrogenase (LDH)/serum LDH ratio greater than 0.6, or an effusion LDH level greater than two-thirds of the upper limit of the laboratory’s reference range of serum LDH (1). Tuberculous pleural effusion was diagnosed if any of the following were present: mycobacterium tuberculosis cultured in pleural fluid, tissue or sputum; acid fast bacilli confirmed in sputum, pleural fluid or tissues; high adenosine deaminase (ADA, over 40 U/L); a radiological suspicion of tuberculous pleuritis. Parapneumonic effusion was diagnosed with clinical evidence of infection (fever, chill, or leukocytosis), respiratory symptoms (cough, sputum, or chest tightness), a high neutrophil count in the pleural effusion, and new or changed infiltrates as observed on chest radiography with or without bacteria cultured in sputum or pleural effusion. Malignant effusion was defined by the presence malignant cells in pleural fluid cytology or a massive unilateral pleural effusion with lung cancer.
Clinical data and pleural fluid analysis
The following clinical data were collected at enrollment: age, gender, and co-morbid conditions. Routine pleural fluid analysis was completed at the time of study enrolment and included glucose, protein, LDH, pH, microscopy, culture, and cytology. The serum and pleural levels of sRAGE were determined using a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kit which was purchased from R&D Systems (Minneapolis, MN, USA).
Statistical analysis
Descriptive data are expressed as medians with interquartile range (IQR), and frequencies are expressed as numbers (%). A chi-squared test was used to compare categorical variables, while continuous variables were compared using a Kruskal-Wallis test. To determine the discriminative power of the various cutoffs of serum and pleural sRAGE, receiver operating characteristic (ROC) curve was drawn and area under the curve (AUC) was calculated. A P value of <0.05 was regarded as statistically significant.
Results
Characteristics of patients and pleural fluid analysis
Sixty-two consecutive patients who had pleural fluid from undiagnosed pleural effusions previously stored were entered into a pleural biomarkers study between January 2013 and January 2015. However, 15 pleural fluid samples were subsequently excluded when the effusion was found to be transudate and undiagnosed. The clinical characteristics of the remaining 47 patients with exudative pleural effusion are detailed in Table 1. Of the total number of patients, 29 were male (62%) and the median age was 63 years (range, 50–79 years). here were 21 patients with a tuberculous effusion, and the groups diagnosed with parapneumonic or malignant effusion comprised 13 patients each. Tuberculosis patients tended to be younger than the other patients, while malignant effusion most often occurred in males. In total, 54% of patients with a malignant effusion were found to have an adenocarcinoma, and there was a higher incidence of fever in patients with a parapneumonic effusion. Table 2 showed laboratory finding of serum and pleural fluid of 47 patients. The serum and pleural fluid white blood cell count, neutrophil fraction, serum C-reactive protein (CRP), and pleural LDH were all higher in patients with pneumonia. The pleural ADA was higher in patients with tuberculous effusion while the pleural carcinoembryonic antigen was higher in those with a malignant effusion. The microorganisms from patients with parapneumonic effusion were Stenotrophomonas maltophilia, Streptococcus constellatus, Providencia rettgeri, Streptococcus intermedius, Klebsiella pneumonia, Escherichia coli in six patients and non-specific pathogen in seven patients.

Full table
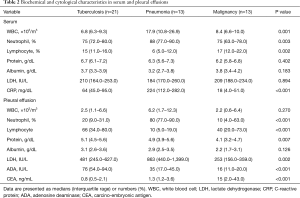
Full table
sRAGE levels in serum and pleural effusion
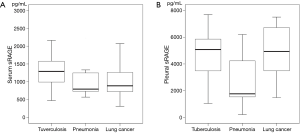
The pleural sRAGE levels (median, 4,595 pg/mL; IQR, 2,278–5,861 pg/mL) were higher than those (median, 1,122 pg/mL; IQR, 759–1,333 pg/mL) of serum (P<0.001). The serum sRAGE levels were significantly elevated in the samples taken from patients with tuberculosis (median, 1,291 pg/mL; IQR, 948–1,711 pg/mL) when compared with both pneumonia (median, 794 pg/mL; IQR, 700–1,255 pg/mL) and lung cancer (median, 886 pg/mL; IQR, 722–1,285 pg/mL) patients (P=0.029; Figure 1A). The pleural sRAGE levels in pneumonia (median, 1,763 pg/mL; IQR, 1,262–4,431 pg/mL) were lower than those in both tuberculosis (median, 5,081 pg/mL; IQR, 3,300–6,004 pg/mL) and lung cancer (median, 4,936 pg/mL; IQR, 3,282–7,018 pg/mL) (P=0.009; Figure 1B).
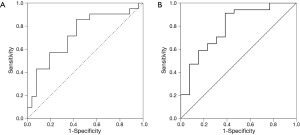
The ROC curve analysis selected 896 pg/mL as the best serum sRAGE level cutoff value for tuberculosis (sensitivity, 86%; specificity, 58%; AUC =0.727; P=0.008) (Figure 2A). For the level of sRAGE in pleural effusion, the ROC curve analysis selected 2,231 pg/mL as the best cutoff value for pneumonia (sensitivity, 91%; specificity, 62%; AUC =0.792; P=0.002) (Figure 2B).
Discussion
Our results demonstrated that the levels of pleural sRAGE of patients with pneumonia were lower compared to those with tuberculosis or lung cancer, whereas the plasma sRAGE levels of patients with tuberculosis were increased compared to those with pneumonia or lung cancer.
To the best of our knowledge, this is the first study to demonstrate an association between the level of sRAGE in pleural effusions and various pulmonary diseases. Because RAGE is a cell surface receptor that is expressed at high levels in the lung and has roles in homeostasis and pro-inflammatory reactions (2,6), we hypothesized that there would be a high level of, and obvious disparity between, sRAGE in the pleural samples of various pulmonary diseases. Furthermore, we expected that the serum and pleural levels of sRAGE in pneumonia might be increased because the high levels of sRAGE in bronchoalveolar lavage samples taken from patients with ARDS and ALI revealed its potential utility as a marker of inflammation and lung damage. As expected, the median pleural level of sRAGE was higher than the sRAGE level in serum samples taken from pulmonary disease patients in this study. This is because of the high levels of RAGE and sRAGE in lung tissue. However, the pleural and serum sRAGE levels for pneumonia were low compared with those for tuberculosis and lung cancer. The engagement of RAGE by its ligands leads to the activation of NF-κB and the mitogen-activated protein kinase (MAPK) pathways (13). In murine model study (6) of RAGE knockout and wild type mice inoculated with S. pneumonia, the pneumonia resulted in an up-regulation of constitutively present RAGE expression in the lung tissue of wild type mice, while RAGE knockout mice showed improved survival accompanied by a lower bacterial load in the lungs. In another study of sRAGE levels during severe sepsis in humans (14), the concentrations of sRAGE did not change during human endotoxemia or infection. Therefore, RAGE or sRAGE are not upregulated in all bacterial infections. Previous studies have shown that plasma sRAGE levels were higher in ARDS and ALI patients compared with controls (8,10,15). Because RAGE plays an important role in modulating the adhesion, migration, and proliferation of alveolar epithelial cells (2,5), upregulation of RAGE or sRAGE may be associated more with lung damage than bacterial infection and an acute inflammation cascade.
Pleural and serum sRAGE levels from patients with tuberculosis were increased compared to patients with pneumonia or lung cancer in our study. In another murine model study (16) with RAGE knockout and wild type mice, pulmonary RAGE expression was increased during tuberculosis in wild type mice while the RAGE knockout mice had a modestly higher mycobacterial load in the lungs after 3 weeks. That study suggested that RAGE plays a beneficial role in the host response to pulmonary tuberculosis. The elevation of RAGE and sRAGE expression in tuberculosis may be associated with its possible role in cell-mediated immunity during chronic inflammation (16).
The serum sRAGE levels of lung cancer patients were lower compared with those of tuberculosis patients. Whereas upregulation of the RAGE expression was observed in most of the primary human tumors examined (17), the RAGE and sRAGE levels were decreased in lung cancer, suggesting their use as markers of diagnosis or prognosis in lung cancer (11,18). Because RAGE plays a role in cell adhesion, migration, and proliferation of lung tissue, the downregulation of RAGE and sRAGE results in differentiation, proliferation, and migration of cancer cells in the lung (11,18). In our study, the sRAGE levels for lung cancer were lower than those for tuberculosis, and this tendency was explained by the carcinogenetic effect of the lower sRAGE state. However, there were not differences of level of sRAGE as types of lung cancer. Although the serum levels of sRAGE from lung cancer were lower in our study, the pleural levels were higher compared with those for pneumonia. The activation and upregulation of RAGE enhances cell survival in cases of cell dysfunction due to increased AGE levels and oxidative stress (19). The formation of pleural effusions in lung cancer or tuberculosis is a chronic process compared with their formation in pneumonia, as a result of an acute bacterial infection. Therefore, greater oxidative stress and chronic inflammation over a long period of time leading to disease progression in tuberculosis or lung cancer may affect the upregulation of the pleural level of sRAGE in a different manner from pneumonia, which is a relatively acute process.
In our study, the sensitivity and specificity of the serum sRAGE for tuberculosis were both 86%, and those of the pleural sRAGE for pneumonia were 91% and 62%, respectively. The diagnostic accuracy of the serum sRAGE level for tuberculosis and the pleural sRAGE level for pneumonia were not particularly high in this study, but consideration of the sRAGE levels would be useful in cases where the cause of a pleural effusion is ambiguous. Especially, in different diagnosis for pleural effusion due to tuberculosis or pneumonia with indefinite level of neutrophil count or ADA, the level of sRAGE could be helpful marker.
There were several limitations to the present study. First, the number of participants was relatively small. A larger sample size of patients with pleural effusion would be necessary to confirm the association between sRAGE and pulmonary disease. Second, the serum and pleural samples used in our study had been stored for several days or months at −70 °C. As reported in previous studies of serum sRAGE (11,12,18), it remains unknown as to whether this extended storage has any effect on the serum and pleural sRAGE levels.
Conclusions
Our study demonstrated that the serum sRAGE levels for tuberculosis were increased compared with those for both lung cancer and pneumonia, while the pleural sRAGE levels for pneumonia were low compared with those for tuberculosis and lung cancer. The sensitivity and specificity of the serum sRAGE for tuberculosis was 86%, and those of the pleural sRAGE for pneumonia were 91% and 62%, respectively. Among patients with exudative effusion, pleural sRAGE and serum sRAGE measurements may be a useful supportive diagnostic tool in the evaluation of ambiguous pleural effusion. The discordant data for sRAGE between serum and pleural effusion for various pulmonary diseases suggests that the sRAGE levels may relate to the chronic process of lung damage and inflammation. Further studies will be needed to validate the role of sRAGE in pulmonary disease, as well as the relationship between RAGE or sRAGE and the pathophysiology of lung damage.
Acknowledgements
Funding: This research was supported by Hallym University Research Fund 2013 (HURF-2013-05).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study received ethical approval (Institutional Review Board number 2012-11-98) from the Research Ethics Committee of Hallym University Kangnam Sacred Heart Hospital, and written informed consent was obtained from all patients.
References
- Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002;346:1971-7. [Crossref] [PubMed]
- Buckley ST, Ehrhardt C. The receptor for advanced glycation end products (RAGE) and the lung. J Biomed Biotechnol 2010;2010:917108.
- Fritz G. RAGE: a single receptor fits multiple ligands. Trends Biochem Sci 2011;36:625-32. [Crossref] [PubMed]
- Liliensiek B, Weigand MA, Bierhaus A, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest 2004;113:1641-50. [Crossref] [PubMed]
- van Zoelen MA, Achouiti A, van der Poll T. The role of receptor for advanced glycation endproducts (RAGE) in infection. Crit Care 2011;15:208. [Crossref] [PubMed]
- van Zoelen MA, Schouten M, de Vos AF, et al. The receptor for advanced glycation end products impairs host defense in pneumococcal pneumonia. J Immunol 2009;182:4349-56. [Crossref] [PubMed]
- Guo WA, Knight PR, Raghavendran K. The receptor for advanced glycation end products and acute lung injury/acute respiratory distress syndrome. Intensive Care Med 2012;38:1588-98. [Crossref] [PubMed]
- Uchida T, Shirasawa M, Ware LB, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 2006;173:1008-15. [Crossref] [PubMed]
- Jabaudon M, Futier E, Roszyk L, et al. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med 2011;39:480-8. [Crossref] [PubMed]
- Gu W, Xu Z, Qi F, et al. Plasma levels of soluble receptor for advanced glycation end products in patients with acute respiratory distress syndrome. Int J Clin Exp Med 2014;7:5558-62. [PubMed]
- Jing R, Cui M, Wang J, et al. Receptor for advanced glycation end products (RAGE) soluble form (sRAGE): a new biomarker for lung cancer. Neoplasma 2010;57:55-61. [Crossref] [PubMed]
- Iwamoto H, Gao J, Pulkkinen V, et al. Soluble receptor for advanced glycation end-products and progression of airway disease. BMC Pulm Med 2014;14:68. [Crossref] [PubMed]
- Bierhaus A, Stern DM, Nawroth PP. RAGE in inflammation: a new therapeutic target? Curr Opin Investig Drugs 2006;7:985-91. [PubMed]
- Achouiti A, Föll D, Vogl T, et al. S100A12 and soluble receptor for advanced glycation end products levels during human severe sepsis. Shock 2013;40:188-94. [Crossref] [PubMed]
- Nakamura T, Sato E, Fujiwara N, et al. Increased levels of soluble receptor for advanced glycation end products (sRAGE) and high mobility group box 1 (HMGB1) are associated with death in patients with acute respiratory distress syndrome. Clin Biochem 2011;44:601-4. [Crossref] [PubMed]
- van Zoelen MA, Wieland CW, van der Windt GJ, et al. Receptor for advanced glycation end products is protective during murine tuberculosis. Mol Immunol 2012;52:183-9. [Crossref] [PubMed]
- Bartling B, Hofmann HS, Weigle B, et al. Down-regulation of the receptor for advanced glycation end-products (RAGE) supports non-small cell lung carcinoma. Carcinogenesis 2005;26:293-301. [Crossref] [PubMed]
- Marinakis E, Bagkos G, Piperi C, et al. Critical role of RAGE in lung physiology and tumorigenesis: a potential target of therapeutic intervention? Clin Chem Lab Med 2014;52:189-200. [Crossref] [PubMed]
- Sparvero LJ, Asafu-Adjei D, Kang R, et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med 2009;7:17. [Crossref] [PubMed]


