- 1Centre for Biomedical Research, School of Medicine, University of Castilla-La Mancha, Albacete, Spain
- 2Hospital General La Mancha Centro, Servicio de Salud de Castilla-La Mancha, Ciudad Real, Spain
- 3Gerencia de Atención Primaria, Salud de Castilla y Leon, Avila, Spain
- 4Gerencia de Emergencias Sanitarias, Salud de Castilla y Leon, Spain
- 5Centre for Biomedical Research, School of Medicine, University of Castilla-La Mancha, Ciudad Real, Spain
SARS-CoV-2 is a new coronavirus that has caused a worldwide pandemic. It causes severe acute respiratory syndrome (COVID-19), which is fatal in many cases, and is characterized by a cytokine release syndrome (CRS). Great efforts are currently being made to block the signal transduction pathway of pro-inflammatory cytokines in order to control this “cytokine storm” and rescue severely affected patients. Consequently, possible treatments for cytokine-mediated hyperinflammation, preferably within approved safe therapies, are urgently being researched to reduce rising mortality. One approach to inhibit proinflammatory cytokine release is to activate the cholinergic anti-inflammatory pathway through nicotinic acetylcholine receptors (α7nAchR). Nicotine, an exogenous α7nAchR agonist, is clinically used in ulcerative colitis to counteract inflammation. We have found epidemiological evidence, based on recent clinical SARS-CoV-2 studies in China, that suggest that smokers are statistically less likely to be hospitalized. In conclusion, our hypothesis proposes that nicotine could constitute a novel potential CRS therapy in severe SARS-CoV-2 patients.
Introduction
SARS-CoV-2 is a new coronavirus that originated in Wuhan (Hubei Province, China) in December 2019, and it has already developed into a pandemic with worldwide spread (1). It causes a severe acute respiratory syndrome (2). SARS-CoV-2 is the third coronavirus outbreak to occur this current century, following severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) (3).
SARS-CoV-2 causes varying degrees of illness degrees. Fever and cough are dominant symptoms, but severe disease also occurs. Then, when COVID-19 patients' aggravation is presented, lung hyperinflammation may occur due to a virus-activated “cytokine storm” or cytokine release syndrome (CRS) (4). Among different cytokines increased in such exacerbated response (5), Interleukin-6 (IL-6) in serum is mainly expected to predict the severity of SARS-CoV-2 pneumonia, as suppression of pro-inflammatory IL-6 have been shown to have a therapeutic effect in many inflammatory diseases, including viral infections (6).
In severe cases, SARS-CoV-2 has been shown to activate both the innate and adaptive immune system in the alveolar tissue, inducing the release of many cytokines and subsequent cytokine release syndrome (7). During this response, levels of pro-inflammatory cytokines (including TNFa, interleukin (IL)-1b, IL-6, and IL-8) are increased (5), which is also an important cause of death (8). Therefore, one may think that controlling such crucial inflammatory factors could be a successful approach to reduce mortality in severe patients.
Could the Cytokine Storm be Controlled by a Physiological Protective Anti-Inflammatory Mechanism?
The existence of a cholinergic anti-inflammatory pathway that modulates inflammatory responses during systemic inflammation has been demonstrated (9). a7-nicotinic acetylcholine receptor (a7nAChR) are known to be expressed on macrophages and to be essential for attenuating the inflammatory response by calcium influx-mediated activation during systemic inflammation (10). The underlying mechanism conveys that activation of a7nAChR on infiltrated inflammatory cells, including macrophages and neutrophils, induces suppression of NF-kB activation (11) and secretion of pro-inflammatory cytokines and chemokines from inflammatory cells, including alveolar macrophages (12). Interestingly, it has been already reported that nicotine, an a7nAChR agonist, exerts an anti-inflammatory effect in a murine model of acute lung injury (13). In fact, in other inflammatory diseases, such as ulcerative Colitis (UC), smoking or treatment with nicotine has demonstrated to significantly decrease the risk of developing the disease (14).
The Nicotine-COVID19 Hypothesis
In this scenario, we hypothesize that nicotine could ameliorate the cytokine storm and severe related inflammatory response through a7nAChR-mediated cholinergic anti-inflammatory pathway. Nicotine is an accessible, existing, and approved treatment, with described side effects, that could likely reduce the rising mortality in the short term.
Support for the Hypothesis
Paradoxically, it is well-stablished that smokers have a significantly increased risk of chronic respiratory disease and acute respiratory infections. Current smokers have a higher risk of developing influenza compared to non-smokers (15). Smoking is also significantly associated with MERS-CoV (16), and there is no clear evidence for SARS-CoV-2 (17).
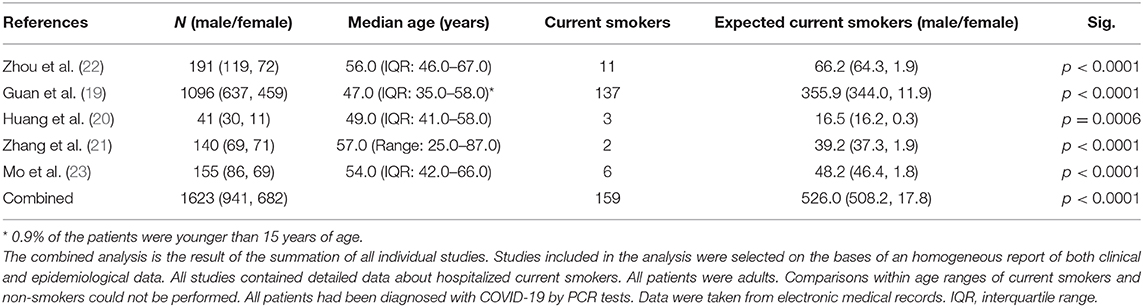
In China, 54.0% of men are current smokers, whereas only 2.6% of women smoke (18); it should therefore be expected that the number of current smokers hospitalized with SARS-CoV-2 should be in a similar or larger percentage with male predominance. However, surprisingly, the number of hospitalized smoking patients in the Chinese outbreak is much lower than expected (19–23). In Table 1 we show results comparing, both separately and using a combined approach, proportions of hospitalized smokers with SARS-CoV-2 in five different studies. Combined total current smokers was 159 compared to the 526·0 expected considering the male/female current smokers' ratio in China. We performed a χ2-test or Fisher's exact test to compare differences between observed and expected current smoker, for all the studies individually and combining all data, and we found significant differences in all cases (p < 0·001). Similar data have been reported for the ongoing pandemic in Europe and America, from patients in Italy (24) and US (25), which strongly support our hypothesis.
On the other hand, there are different formulations for nicotine administration: gums, patches, inhalators, nasal and oral sprays, sublingual tablets, and electronic cigarettes. Apart from the electronic cigarettes, which are quite new, the previous options are considered relatively safe, as most side effects are mild (26). For instance, in the clinical practice transdermal nicotine is administered at high tolerated dosage for controlling clinical manifestations of chronic UC. For acute SARS-CoV-2 treatment to ameliorate hyperinflammation, nicotine dosage and pharmaceutical form could be chosen according to previous experience with UC (14, 27). In addition to nicotine, it has been suggested that both selective α7nAChR agonists and allosteric modulators may be potential tools for the treatment of acute lung injury (12, 28, 29).
To our knowledge, no clinical trials of nicotine in COVID19 patients are currently being run. The most promising trial under run is the one using Tocilizumab, a blocker of IL-6 receptor for the treatment of cytokine storm (6), so it is expected to be an effective drug of severe patients.
To further complete present hypothesis, factors such as age, smoking habits (vg. nicotine administration route), or any others that could influence smoker's health and susceptibility to the infection should be considered in future studies.
Conclusions
It has been observed that the number of current smokers hospitalized in the SARS-CoV-2 outbreak in China is lower than expected when compared to the prevalence of smoking in this country. It has been described that, due to a cytokine storm, many patients are aggravated, showing severe inflation in the lungs. Our proposal may be easily tested in the short term, as it takes advantage of the existence of a cholinergic anti-inflammatory pathway that physiologically modulates inflammatory responses during systemic inflammation effect. Controlling the cytokine storm using nicotinic administration could then be expected to become a new method for the treatment of severe patients, as it has already been proved in UC patients.
Just very recently, a group of recognized experts in the field recommended the “identification and treatment of hyperinflammation using existing, approved therapies with proven safety profiles to address the immediate need to reduce the rising mortality” (8). Current treatment with Tocilizumab seems to be useful to control cytokines storm. However, very strict criteria for its clinical use limits its availability, mainly due to price and adverse effects. Our proposal, recently supported by different non peer-reviewed additional reports (30, 31), suggest that the treatment with nicotine by using any of the already approved pharmaceutical forms available in the market could reduce the lung inflammatory response by controlling the cytokines storm and, therefore, the number of patients in need of hospitalization for aggravation in the SARS-CoV-2 outbreak.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
JG-R and AN designed the study and collected data. JG-R, LJ-D, JN-L, and AN wrote the manuscript. All authors analyzed and interpreted the data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by University of Castilla-La Mancha Research Programme 2020-GRIN-28705.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Isabel Najera for helpful comments that greatly improved the manuscript. This manuscript was released as Pre-print at https://osf.io/chd2k/ on 2020/04/16 that has not been peer-reviewed (32).
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. (2003) 348:1953–66. doi: 10.1056/NEJMoa030781
3. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. (2019) 17:181–92. doi: 10.1038/s41579-018-0118-9
4. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. (2020) 46:846–8. doi: 10.1007/s00134-020-06028-z
5. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. (2014) 124:188–95. doi: 10.1182/blood-2014-05-552729
6. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. (2020) 29:105954. doi: 10.1016/j.ijantimicag.2020.105954
7. Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin Immunol Orlando Fla. (2020) 214:108393. doi: 10.1016/j.clim.2020.108393
8. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. (2020) 395:1033–4. doi: 10.1016/S0140-6736(20)30628-0
9. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. (2000) 405:458–62. doi: 10.1038/35013070
10. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. (2003) 421:384–8. doi: 10.1038/nature01339
11. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. (2017) 2:17023. doi: 10.1038/sigtrans.2017.23
12. Yamada M, Ichinose M. The cholinergic anti-inflammatory pathway: an innovative treatment strategy for respiratory diseases and their comorbidities. Curr Opin Pharmacol. (2018) 40:18–25. doi: 10.1016/j.coph.2017.12.003
13. Mabley J, Gordon S, Pacher P. Nicotine exerts an anti-inflammatory effect in a murine model of acute lung injury. Inflammation. (2011) 34:231–7. doi: 10.1007/s10753-010-9228-x
14. Gomes JP, Watad A, Shoenfeld Y. Nicotine and autoimmunity: the lotus' flower in tobacco. Pharmacol Res. (2018) 128:101–9. doi: 10.1016/j.phrs.2017.10.005
15. Lawrence H, Hunter A, Murray R, Lim WS, McKeever T. Cigarette smoking and the occurrence of influenza - Systematic review. J Infect. (2019) 79:401–6. doi: 10.1016/j.jinf.2019.08.014
16. Alraddadi BM, Watson JT, Almarashi A, Abedi GR, Turkistani A, Sadran M, et al. Risk factors for primary middle east respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis. (2016) 22:49–55. doi: 10.3201/eid2201.151340
17. Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. (2020) 8:e20. doi: 10.1016/S2213-2600(20)30117-X
18. Liu S, Zhang M, Yang L, Li Y, Wang L, Huang Z, et al. Prevalence and patterns of tobacco smoking among Chinese adult men and women: findings of the 2010 national smoking survey. J Epidemiol Community Health. (2017) 71:154–61. doi: 10.1136/jech-2016-207805
19. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708-20. doi: 10.1101/2020.02.06.20020974
20. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
21. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. (2020). doi: 10.1111/all.14238
22. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond Engl. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
23. Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. (2020) 2020:ciaa270. doi: 10.1093/cid/ciaa270
24. Colombi D, Bodini FC, Petrini M, Maffi G, Morelli N, Milanese G, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. (2020) 238:321–9. doi: 10.1148/radiol.2020201433
25. Chow N, Fleming-Dutra K, Gierke R, Hall A, Hughes M, Pilishvili T, et al. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:382–6. doi: 10.15585/mmwr.mm6913e2
26. Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. (2012) 11:CD000146. doi: 10.1002/14651858.CD000146.pub4
27. Sandborn WJ, Tremaine WJ, Offord KP, Lawson GM, Petersen BT, Batts KP, et al. Transdermal nicotine for mildly to moderately active ulcerative colitis - A randomized, double-blind, placebo-controlled trial. Ann Intern Med. (1997) 126:364–71. doi: 10.7326/0003-4819-126-5-199703010-00004
28. Thomsen MS, Mikkelsen JD. The α7 nicotinic acetylcholine receptor complex: one, two or multiple drug targets? Curr Drug Targets. (2012) 13:707–20. doi: 10.2174/138945012800399035
29. Papke RL, Lindstrom JM. Nicotinic acetylcholine receptors: conventional and unconventional ligands and signaling. Neuropharmacology. (2020) 168:108021. doi: 10.1016/j.neuropharm.2020.108021
30. Farsalinos K, Niaura R, Le Houezec J, Barbouni A, Tsatsakis A, Kouretas D, et al. Editorial: nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol Rep. (2020) 7:658–63. doi: 10.1016/j.toxrep.2020.04.012
31. Changeux J-P, Amoura Z, Rey F, Miyara M. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. Qeios. (2020) doi: 10.32388/FXGQSB. [Epub ahead of print].
Keywords: cholinergic anti-inflammatory pathway, nicotine, Cytokine Release Syndrom (CRS), SARS-CoV-2 (virus), COVID- 19, lung
Citation: Gonzalez-Rubio J, Navarro-Lopez C, Lopez-Najera E, Lopez-Najera A, Jimenez-Diaz L, Navarro-Lopez JD and Najera A (2020) Cytokine Release Syndrome (CRS) and Nicotine in COVID-19 Patients: Trying to Calm the Storm. Front. Immunol. 11:1359. doi: 10.3389/fimmu.2020.01359
Received: 27 April 2020; Accepted: 28 May 2020;
Published: 11 June 2020.
Edited by:
Haichao Wang, Feinstein Institute for Medical Research, United StatesReviewed by:
Alain Simard, Northern Ontario School of Medicine, CanadaJens D. Mikkelsen, Rigshospitalet, Denmark
Outi S. Salminen, University of Helsinki, Finland
Copyright © 2020 Gonzalez-Rubio, Navarro-Lopez, Lopez-Najera, Lopez-Najera, Jimenez-Diaz, Navarro-Lopez and Najera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lydia Jimenez-Diaz, lydia.jimenez@uclm.es; Juan D. Navarro-Lopez, Juan.Navarro@uclm.es; Alberto Najera, alberto.najera@uclm.es
†These authors have contributed equally to this work
 Jesus Gonzalez-Rubio
Jesus Gonzalez-Rubio Carmen Navarro-Lopez2
Carmen Navarro-Lopez2 Lydia Jimenez-Diaz
Lydia Jimenez-Diaz Juan D. Navarro-Lopez
Juan D. Navarro-Lopez Alberto Najera
Alberto Najera