Article Text
Abstract
Objectives To examine whether baseline anti-cyclic citrullinated peptide-2 (CCP2) antibody status and concentration correlated with clinical outcomes in patients treated with abatacept or adalimumab on background methotrexate (MTX) in the 2-year AMPLE (Abatacept versus adaliMumab comParison in bioLogic-naïvE rheumatoid arthritis subjects with background MTX) study.
Methods In this exploratory analysis, anti-CCP2 antibody concentration was measured at baseline, and antibody-positive patients were divided into equal quartiles, Q1–Q4, representing increasing antibody concentrations. Clinical outcomes analysed by baseline anti-CCP2 status and quartile included change from baseline in disease activity and disability and remission rates.
Results Baseline characteristics were generally comparable across quartiles and treatment groups. In both treatment groups, anti-CCP2 antibody-negative patients responded less well than antibody-positive patients. At year 2, improvements in disease activity and disability and remission rates were similar across Q1–Q3, but were numerically higher in Q4 in the abatacept group; in contrast, treatment effects were similar across all quartiles in the adalimumab group.
Conclusions In AMPLE, baseline anti-CCP2 positivity was associated with a better response for abatacept and adalimumab. Patients with the highest baseline anti-CCP2 antibody concentrations had better clinical response with abatacept than patients with lower concentrations, an association that was not observed with adalimumab.
Trial registration number NCT00929864.
- Ant-CCP
- Autoantibodies
- Rheumatoid Arthritis
- DMARDs (biologic)
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Video abstract
Introduction
The introduction of multiple biologic disease-modifying antirheumatic drugs (DMARDs) and one new targeted synthetic DMARD has significantly improved rheumatoid arthritis (RA) treatment. However, better predictors of treatment response in individual patients are still needed.
Anti-citrullinated protein antibodies (ACPA) are a sensitive and highly specific marker of RA1 and have been incorporated into the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) diagnostic criteria.2 ACPA are present many years prior to the onset of clinical RA in many at-risk individuals, and 70%–80% of patients with RA are ACPA positive.3 As clinical disease develops, ACPA concentration increases, the number of recognised epitopes expands and isotype usage evolves.4 ,5 ACPA may also predict a more severe disease course with more erosive disease6; however, the clinical relevance of ACPA concentration is unclear.7 The relationship between ACPA status/concentration and response to therapy has not been elucidated but is of interest.8
In the clinic, ACPA can be detected using anti-cyclic citrullinated peptide (CCP) ELISA, such as the CCP2 assay.9 Here, we examined whether baseline anti-CCP2 IgG status and concentration influenced clinical outcomes in patients treated with abatacept or adalimumab in the head-to-head, 2-year AMPLE (Abatacept versus adaliMumab comParison in bioLogic-naïvE RA subjects with background methotrexate (MTX)) study.10 ,11 AMPLE provided a unique opportunity to explore baseline anti-CCP2 concentration as a predictor of response to two therapies with different mechanisms of action.
Methods
Study design
AMPLE (NCT00929864) was a 2-year, phase IIIb, randomised, investigator-blinded study. Biologic-naïve patients with active RA and an inadequate response to MTX were randomised to 125 mg subcutaneous abatacept weekly or 40 mg adalimumab bi-weekly, both on background MTX.10 ,11
ACPA analysis
Baseline anti-CCP2 antibody status (positive/negative) and concentration were determined using an anti-CCP2 IgG ELISA (Euro Diagnostica Immunoscan CCPlus, Malmö, Sweden; obtained from IBL America). Patients with a baseline anti-CCP2 IgG concentration of ≥25 AU/mL were considered to be positive and were further divided into equal quartiles according to concentration (Q1–Q4 (highest concentration)).
Outcome measures
Efficacy outcomes up to day 729 were assessed according to baseline anti-CCP2 IgG status and concentration quartile. Outcomes were adjusted mean change from baseline in Disease Activity Score 28 (C reactive protein; DAS28 (CRP)) and Health Assessment Questionnaire Disability Index (HAQ-DI) over time, percentage of patients achieving DAS28 (CRP) <2.6, ACR/EULAR remission rates defined by Clinical Disease Activity Index (CDAI; ≤2.8) or Simplified Disease Activity Index (SDAI; ≤3.3) criteria and ACR 50/70 response rates.
Statistical analyses
Analyses included all randomised and treated patients. Adjusted mean change from baseline for DAS28 (CRP) and HAQ-DI was determined for each time point using analysis of covariance (ANCOVA), with treatment and baseline DAS28 (CRP) stratification as factors and baseline values as a covariate. For comparisons between Q1–Q3 and Q4, anti-CCP2-negative and Q4, and abatacept Q4 and adalimumab Q4, the adjusted mean difference was estimated using an ANCOVA model with treatment, quartile, treatment by quartile interaction and baseline DAS28 (CRP) stratification as factors and baseline values as a covariate.
Results
Patient disposition and baseline characteristics
In AMPLE, 646 patients were randomised (abatacept, n=318; adalimumab, n=328), of whom 252 (79.2%) abatacept-treated and 245 (74.7%) adalimumab-treated patients completed year 2.10 Serum samples were available from 508 patients at baseline: 120 (23.6%) were anti-CCP2 negative and 388 (76.4%) were anti-CCP2 positive. The number of patients per treatment group was similar in each anti-CCP2 quartile, with no consistent differences in baseline characteristics across anti-CCP2 quartiles or treatment groups (table 1 and see supplementary table S1). Quartile limits are shown in table 1.
Patient demographics and baseline characteristics
Mean change from baseline in disease activity and disability
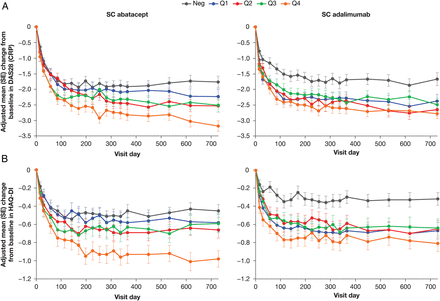
Although at least a ‘moderate’ EULAR response was observed in both treatment groups, improvements in DAS28 (CRP) were significantly less pronounced in patients who were anti-CCP2 negative at baseline than in those who were anti-CCP2 positive (figure 1A). The mean improvement in DAS28 (CRP) at day 729 for abatacept was significantly greater in Q4 than in Q1–Q3 combined (adjusted mean treatment difference (AMTD) (95% CI) Q1–Q3 vs Q4: –0.69 (–1.15 to –0.23); p=0.003), whereas in the adalimumab group, improvement was similar across all quartiles (AMTD (95% CI) Q1–Q3 vs Q4: –0.21 (–0.64 to 0.23); p=0.358). The AMTD (95% CI) for abatacept Q4 versus adalimumab Q4 was –0.45 (–1.00 to 0.10; p=0.112).
(A) Adjusted mean (SE) change from baseline in Disease Activity Score 28 (C reactive protein; DAS28 (CRP)) by baseline cyclic citrullinated peptide-2 (CCP2)-IgG status and quartile. Estimated mean treatment difference at day 729 for anti-CCP2 quartile (Q)1–Q3 combined vs Q4: subcutaneous (SC) abatacept p=0.003, SC adalimumab p=0.358; for anti-CCP2 negative (Neg) vs Q4: SC abatacept p<0.0001, SC adalimumab p=0.0006. (B) Adjusted mean (SE) change from baseline in Health Assessment Questionnaire Disability Index (HAQ-DI) by baseline CCP2-IgG status and quartile. Estimated mean treatment difference at day 729 for Q1–Q3 combined vs Q4: SC abatacept p=0.021, SC adalimumab p=0.735; for Neg vs Q4: SC abatacept p=0.002, SC adalimumab p=0.005. Adjusted mean changes from baseline were determined for each time point by analysis of covariance, with treatment and DAS28 (CRP) stratification as factors and baseline values as a covariate. Number of patients in each quartile group: Q1 (28–235)=97; Q2 (236–609)=97; Q3 (613–1046)=97; Q4 (1060–4894)=97; Neg (<25)=120.
A similar pattern was seen for HAQ-DI: mean changes from baseline were smallest in patients who were anti-CCP2 negative at baseline in both treatment groups (figure 1B). Mean change from baseline in HAQ-DI was similar across all anti-CCP2 quartiles for adalimumab, but significantly greater in abatacept Q4 than in Q1–Q3. The AMTD (95% CI) for Q1–Q3 versus Q4 for abatacept was –0.24 (–0.44 to –0.04; p=0.021), and for adalimumab was –0.03 (–0.23 to 0.16; p=0.735). The AMTD (95% CI) for abatacept Q4 versus adalimumab Q4 was –0.17 (–0.41 to 0.07; p=0.173).
Remission rates, DAS28 (CRP) <2.6 and ACR response rates
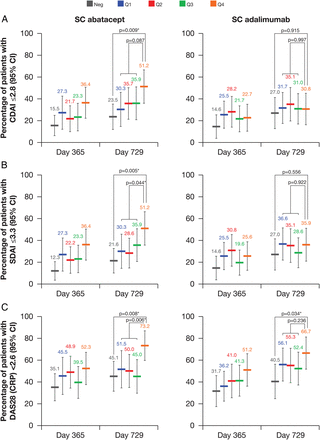
In both treatment groups, the percentage of patients achieving CDAI or SDAI remission or DAS28 (CRP) <2.6 was lower in the baseline anti-CCP2 antibody-negative subgroup than in the anti-CCP2 antibody-positive subgroup (figure 2). The percentages of patients achieving CDAI (figure 2A) and SDAI (figure 2B) remission were highest in Q4 versus Q1–Q3 in the abatacept, but not the adalimumab, treatment group at days 365 and 729; however, the percentage of patients achieving DAS28 (CRP) <2.6 was highest in Q4 for both abatacept and adalimumab (figure 2C). The trend in the anti-CCP2 interquartile differences was not as clear for ACR response rates (data not shown).
Percentage (95% CI) of patients achieving (A) Clinical Disease Activity Index (CDAI) remission rates by baseline cyclic citrullinated peptide-2 (CCP2)-IgG status and quartile. (B) Simplified Disease Activity Index (SDAI) remission rates by baseline CCP2-IgG status and quartile. (C) Disease Activity Score 28 (C reactive protein; DAS28 (CRP)) <2.6 by baseline CCP2-IgG status and quartile. p Values indicate the statistical significance of the estimated mean treatment difference at day 729 for anti-CCP2 quartile (Q)1–Q3 combined vs Q4 and for anti-CCP2 negative (Neg) vs Q4. Asterisks indicate p values ≤0.05. Number of patients in each quartile group: Q1 (28–235)=97; Q2 (236–609)=97; Q3 (613–1046)=97; Q4 (1060–4894)=97; Neg (<25)=120. SC, subcutaneous.
Overall, CDAI and SDAI remission rates tended to be higher at day 729 than at day 365 regardless of treatment, anti-CCP2 status or concentration. At day 729, CDAI and SDAI remission rates were highest in the abatacept Q4 group.
Discussion
Both adalimumab and abatacept were more effective in patients who were anti-CCP2 positive than in those who were anti-CCP2 negative at baseline. However, there were differences in the pattern of response to the two treatments when assessed by baseline antibody concentration: abatacept treatment effects were more pronounced in the highest anti-CCP2 quartile than in lower quartiles, whereas this effect was not consistently demonstrated for adalimumab. Interquartile differences for abatacept were most prominent when assessed with continuous measures such as DAS28 (CRP) and HAQ-DI versus binary response measures, possibly due to the increased sensitivity to change of continuous measures.
The improved clinical efficacy in anti-CCP2-positive versus anti-CCP2-negative patients for both adalimumab and abatacept suggests that ACPA status may be a relevant factor in predicting treatment response. In contrast to our results, previous studies have suggested that seropositive patients respond less well to tumour necrosis factor (TNF) inhibition than seronegative patients, or that response correlates inversely with autoantibody concentration.12–14 High baseline rheumatoid factor (RF) IgA concentration has been associated with poor response to TNF inhibitor therapy.15 The disparities between studies could be due to differences in patient populations, study setting or ACPA assay used; further study of predictive factors of treatment response in RA with standardisation of biomarker assays is warranted.
The reason for the observed differential pattern of response across quartiles for abatacept and adalimumab is unknown, but may be related to their different mechanisms of action. Abatacept selectively modulates T-cell costimulation and autoantibody production via interaction with B cells, whereas adalimumab binds directly to TNF-α. The B-cell inhibitor rituximab has also been shown to be more effective in patients with RA who are ACPA or RF seropositive versus seronegative.16 In this analysis, 90% of patients in Q4 were also RF positive at baseline; in registry and cohort studies, better abatacept efficacy and retention has been found to be associated with double-positivity or higher ACPA concentration.17–20 Importantly, our analysis was a within-study comparison of two treatments, removing causes of variation inherent in cross-study comparisons.
The trend for continued improvement in remission rates, particularly in the Q4 abatacept group, may suggest that the effects of costimulatory blockade increases over time. The clinical relevance of this observation is unknown.
This exploratory analysis has some inherent limitations. Baseline serum samples were not available for all patients and there were differences in several baseline characteristics between groups (although differences were not consistent across quartiles or treatment groups). As the analysis was not preplanned, patients were not stratified by anti-CCP2 concentration at randomisation and lack of blinding in AMPLE may have influenced the patient-reported outcome measures. Additionally, there is no standard, universally accepted ACPA assay and so findings may have differed with an alternative assay. The anti-CCP2 ELISA used here demonstrated relative linearity across the assay standards and based on the distribution of measured concentrations in the anti-CCP2-positive population.
In this exploratory analysis from the AMPLE study, treatment effects for both abatacept and adalimumab were greater in patients who were anti-CCP2 positive at baseline than in those who were anti-CCP2 negative. Higher baseline anti-CCP2 concentration correlated with better DAS28 (CRP) and HAQ-DI responses and greater CDAI and SDAI remission rates with abatacept but not with adalimumab (both on background MTX). The prognostic and predictive value of ACPA status and concentration in RA, however, needs to be further examined beyond this exploratory analysis to improve our understanding of the heterogeneity in response and inform treatment decision making in the clinic.
Acknowledgments
Professional medical writing and editorial assistance was provided by Andreas Sakka at Caudex and was funded by Bristol-Myers Squibb. Sandra Overfield (Clinical Operations, BMS) managed the study execution and sample collection. Lauren J Lahey, Chris Rhodes and Catriona A Wagner (VA Palo Alto Health Care System and Stanford University) assisted with data collection, including sample handling and performing anti-CCP2 ELISA testing.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1 - Online supplement
Footnotes
Handling editor Tore K Kvien
Contributors JS designed and conducted the majority of experiments, interpreted the data and assisted in manuscript preparation. MS, RF, MEW and WHR were involved in the study design and interpretation of data and assisted in the preparation of the manuscript. SEC and AJ provided input into the study design and were involved in analysis and interpretation of data and manuscript preparation. JZ conducted the statistical analyses and assisted in manuscript preparation. MAM contributed to the design of the study, provided input into the interpretation of data and assisted in manuscript preparation. SP was involved in study design, interpretation of data and contributed to manuscript preparation.
Funding This study was funded by Bristol-Myers Squibb.
Competing interests JS received research funding support from Bristol-Myers Squibb. MS received consultancy fees from AbbVie, Amgen, Antares, Bristol-Myers Squibb, Eli Lilly, Horizon, Johnson & Johnson, Pfizer, Roche and UCB; served on speakers bureaus for AbbVie; and received research funding from UCB. RF received grant/research support from AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Celgene, Genzyme, Janssen, Eli Lilly, Merck, Novartis, Pfizer, Roche, Sanofi Aventis and UCB; and acted as a consultant for AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Janssen, Eli Lilly, Pfizer, Roche, Sanofi-Aventis and UCB. MEW received consulting fees from Bristol-Myers Squibb, Crescendo Bioscience, UCB, AbbVie, Roche, Janssen, Pfizer, Lilly, Amgen and Novartis; and received grant support from Bristol-Myers Squibb, Crescendo Bioscience and UCB. SEC, MAM and SP are employees of and hold stock in Bristol-Myers Squibb. AJ and JZ are employees of Bristol-Myers Squibb. WHR has no conflicts of interest to report.
Ethics approval The protocol was approved by the institutional review boards and independent ethics committees at the participating sites.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement ACR response data available from authors upon request.