Article Text
Abstract
Objective SLE is a chronic inflammatory autoimmune disease characterised by the excessive production of autoantibodies, immune complexes and proinflammatory cytokines. Repository corticotropin injection (RCI) is a naturally sourced complex mixture of adrenocorticotropic hormone analogues and other pituitary peptides. RCI is approved by the US Food and Drug Administration for use during an exacerbation or as maintenance therapy in select cases of SLE. This paper discusses the design and baseline characteristics of a multicentre, double-blind, randomised, placebo-controlled, 24-week clinical trial evaluating the effect of RCI in reducing disease activity for patients with persistently active SLE despite moderate-dose corticosteroid use.
Methods Efficacy will be evaluated using the SLE Responder Index-4 (SRI-4), SLE Disease Activity Index-2000 (SLEDAI-2K), British Isles Lupus Assessment Group-2004 (BILAG-2004) and Physician’s Global Assessment (PGA). The primary efficacy endpoint will be the proportion of SRI-4 responders at week 16. Secondary and exploratory endpoints will include changes in disease activity scores over time, prednisone dose and biomarkers of inflammation and bone turnover. The safety and tolerability profile of RCI will also be evaluated through adverse event profiles, physical examination, clinical laboratory tests and serum cortisol levels.
Results Target enrolment for this global study is 270 patients, and as of 15 November 2019, the modified intent-to-treat population included 169 patients. The study cohort had 91.7% women, had a mean age of 39.7 years, mean SLEDAI-2K total score of 9.9, mean BILAG-2004 total score of 18.1, mean PGA of 59.7 and mean prednisone or equivalent daily dose of 11.1 mg. A total of 79.3% and 64.5% of patients were receiving concomitant antimalarial or immunosuppressive therapy, respectively.
Conclusions Data from this study will provide valuable insights into the therapeutic role of RCI in refractory SLE, as well as important information regarding its safety profile.
- SLE
- autoimmune diseases
- inflammation
- corticosteroids
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
SLE, a chronic autoimmune disease, affects multiple organs and has diverse clinical manifestations.1 The goals of SLE treatment are to reduce disease activity and prevent the accrual of damage from either the disease itself or the medications used to treat the disease.2 Corticosteroids, a mainstay of therapy, are a prominent cause of bone loss,3–5 as well as damage to other organs.6 7 Treatments for persistently active SLE that safely reduce patient dependence on chronic corticosteroid use are needed,8 but the complex nature of the disease has made drug development difficult.
Repository corticotropin injection (RCI; Acthar Gel) is approved by the US Food and Drug Administration for use during an exacerbation or as maintenance therapy in selected cases of SLE.9 This naturally sourced complex mixture of purified adrenocorticotropic hormone analogues and other pituitary peptides achieves both its steroid-dependent as well as steroid-independent anti-inflammatory effects through melanocortin receptors.10 11 RCI binds to melanocortin receptor 2 (MC2R) to stimulate steroid hormone secretion from the adrenal cortex.11 Its additional anti-inflammatory effects are produced via direct activation of MC1R, MC3R, MC4R or MC5R melanocortin receptors expressed by B and T cells, as well as in a variety of tissues often affected in SLE.10–15 These effects include the inhibition of lymphocyte migration in the skin, lung, heart, kidney, liver and joints;13–15 reductions in the expression and secretion of proinflammatory interleukin (IL) 1, IL-6, IL-8, interferon-γ, tumour necrosis factor-α, IL-2 and IL-17 and adhesion molecules, including intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and E-selectin11 13; and increases in regulatory T (Treg) cells and IL-10.11 Animal studies and evidence in humans have suggested that the binding of RCI to MC2R expressed in osteoblasts may also have a protective effect against the bone loss induced by corticosteroids.16 17 This binding stimulates vascular endothelial growth factor production by osteoblasts to support the maturation and survival of these cells, as reflected by reductions in bone resorption markers and increases in bone formation markers.15 17
Findings from a pilot study suggested that RCI used with moderate-dose corticosteroids (prednisone 7.5 to 30 mg/day or equivalent), as well as antimalarials or immunosuppressants, is safe and well tolerated and may be effective for patients with persistently active SLE with rash or arthritis.18 19 Significant benefits of RCI compared with placebo were observed in secondary analyses of total hybrid SLE Disease Activity Index (hSLEDAI), total British Isles Lupus Assessment Group-2004 (BILAG-2004) and Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) scores at week 8; benefits were also significant in post hoc analyses of the SLE Responder Index-4 (SRI-4) at week 8.18 The hSLEDAI is identical to the Safety of Estrogens in Lupus National Assessment-SLEDAI used in clinical trials for belimumab19–22 but uses the proteinuria definition from the SLE Disease Activity Index-2000 (SLEDAI-2K). In a separate post hoc analysis of these pilot study data, treatment with RCI demonstrated sustained improvements in disease activity over the 52-week study period.23 These results provide a rationale for further clinical investigation of RCI for the treatment of persistently active SLE in larger studies evaluating validated response measures, such as the SRI-4. Here, we describe the design and baseline patient characteristics of this multicentre, randomised, double-blind, placebo-controlled trial.
Study objectives
The primary objective of this study is to provide additional data regarding the efficacy and safety of RCI in patients with SLE requiring moderate-dose corticosteroids. Exploratory objectives include examining pharmacodynamic and steroid-independent effects of RCI on lymphocyte recruitment, inflammatory cytokines and bone turnover.
Methods
Study overview
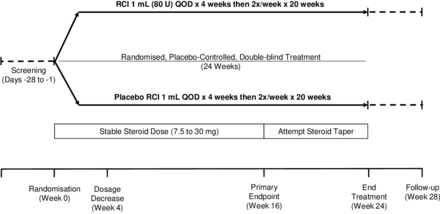
This prospective, multicentre, randomised, double-blind, parallel-group, placebo-controlled study will enrol subjects across approximately 60 study centres worldwide over a 3-year period. A schematic representation of the study design is presented in figure 1. Patients with persistently active SLE and moderate to severe rash and/or arthritis, despite receipt of moderate-dose corticosteroids and allowed standard of care, will enter a screening period of up to 28 days before being randomly assigned to receive RCI 1 mL (80 U) or matching placebo for 24 weeks. Between weeks 16 and 24, patients will be encouraged to taper their corticosteroid dose. A follow-up period will occur through 28±5 days after the last dose of study treatment.
Study schematic. QOD, every other day; RCI, repository corticotropin injection.
Protocol registrations and patient consent
The trial protocol is registered at ClinicalTrials.gov (NCT02953821). Written informed consent from the patient is a requirement of enrolment.
Patient population and eligibility
Patients (male or female) who are at least 18 years of age and have persistently active SLE (≥4 weeks before screening) despite the use of moderate-dose corticosteroids (prednisone or prednisone equivalent, 7.5 to 30 mg/day) are eligible for enrolment. Patients are permitted to enrol if on stable doses of an antimalarial, non-steroidal anti-inflammatory drugs (NSAIDs), methotrexate, azathioprine or mycophenolate mofetil. Subjects must have active SLE as demonstrated by a SLEDAI-2K score ≥6 at screening and a clinical SLEDAI-2K (excluding laboratory results) score ≥4 at screening and randomisation. SLEDAI-2K points for arthritis and/or rash must be present at both screening and randomisation. Subjects must have moderate to severe rash or arthritis as demonstrated by BILAG-2004 scores A or B in the mucocutaneous or musculoskeletal domains at both screening and randomisation. Patients will be excluded from enrolment if they have severe renal dysfunction (serum creatinine >2.5 mg/dL or protein creatinine ratio >1.5 g/g), have active central nervous system manifestations of lupus within 3 months before screening or prior to first dose of study drug, or receive medications not permitted for use during the study period. These and other key entry and exclusion criteria are detailed in table 1.
Key inclusion and exclusion criteria
Stratification, randomisation and blinding
Patients determined to be eligible for entry into the study will be randomly assigned in a 1:1 ratio to receive RCI 1 mL (80 U) or volume-matched placebo (block size, 4). Randomisation will be stratified by prednisone equivalent dose (≤20 mg/day vs >20 mg/day) and location (USA vs ex-USA), with each stratum having a separate computer-generated randomisation scheme. Both patients and investigators will be blinded to study treatment assignments.
Interventions
The RCI and placebo treatments will be supplied in kits as 5 mL vials, with the active study drug vials containing 80 U of RCI per millilitre. Before study treatment initiation, patients or their caregivers will be trained on appropriate administration techniques. Patients or caregivers will complete study diary entries recording all study drug administrations and will bring the diary and all used vials to each visit. Each time the study drug is dispensed, compliance will be encouraged, and study diary entries will be reviewed.
Treatment is to be administered subcutaneously every other day for 4 weeks and then twice per week for an additional 20 weeks. Study staff will supervise the administration of the first dose of treatment in the clinic by the patient or the patient’s caregiver and will monitor the patient for allergic or anaphylactic reactions for at least 1 hour post dose. Subsequent doses will be administered at the patient’s home.
Concomitant medication use
Patients must remain on stable daily doses of topical or inhaled corticosteroids through week 16 of the study. Tapering of corticosteroids will be encouraged between weeks 16 and 24 when clinically appropriate and at the discretion of the investigator (figure 1). Antimalarials and NSAIDs will be permitted for patients who have been receiving a stable dose for at least 4 weeks before screening. Methotrexate, azathioprine and mycophenolate mofetil will be permitted for those who have been receiving a stable dose for at least 8 weeks before screening. Doses of background antimalarials, NSAIDs and immunosuppressives must remain constant through study completion.
Study endpoints
Primary and secondary efficacy outcomes (figure 2) will be evaluated using the SRI-4 and changes from baseline in SLEDAI-2K, BILAG-2004, Physician’s Global Assessment (PGA), CLASI and 28-joint count scores. Total scores for the BILAG-2004 Index were calculated using the coding scheme of A=12, B=8, C=1 and D/E=0.24 Exploratory endpoints and the safety and tolerability profile of RCI will also be assessed (figure 2).
Study endpoints. aP values will be derived using Pearson’s χ2 test or Fisher’s exact test, as appropriate. bAnalysis of covariance models will be used, with treatment group as a factor and baseline value as a covariate; mixed models with repeated measurement will be performed as necessary. BILAG-2004, British Isles Lupus Assessment Group-2004; CLASI, Cutaneous Lupus Erythematosus Disease Area and Severity Index; PGA, Physician’s Global Assessment; QOL, quality of life; SFI, Safety of Estrogens in Lupus Erythematosus National Assessment Flare Index; SLEDAI-2K, SLE Disease Activity Index-2000; SRI-4, SLE Responder Index-4; WPAI, Work Productivity and Activity Impairment.
Statistical methods
The primary analysis set for comparing the efficacy of RCI and placebo will be the modified intent-to-treat (mITT) population, defined as all randomised patients who receive at least one dose of study treatment and who provide any postbaseline efficacy data. Safety and tolerability will be assessed for the safety population, defined as all patients who receive one or more doses of study treatment.
Enrolment is expected to yield 270 patients screened, with 169 patients randomised at 60 global study sites. A sample size of 160 patients (80 per treatment group) was determined to provide 90% power to demonstrate statistical significance for the primary endpoint, assuming SRI-4 response rates of 30% in the placebo group and 55% in the RCI group, a significance level of 0.05, and exclusion of two patients who may not qualify for the mITT population after randomisation.
For categorical variables including the primary endpoint, p-values will be derived using Pearson’s χ2 test or Fisher’s exact test, as appropriate. Fisher’s exact test will be used if the responder or non-responder count falls to 5 or lower in either treatment group. For continuous variables, analysis of covariance models will be used, with treatment group as a factor and baseline value as a covariate. Mixed models with repeated measurements will be performed as necessary. All statistical tests will be two-sided, with p-values <0.05 considered statistically significant, and will be performed using SAS V.9.2 (SAS Institute, Inc., Cary, North Carolina, USA) or higher.
Correlations between clinical scores are determined by Pearson correlation coefficients. Statistical significance is determined by t-test.
Results
This trial was initiated on 13 October 2016, and 169 patients have been included in the mITT population as of 15 November 2019. Approximately 92% of the patients are women. The study cohort had a mean age of 39.7 years, mean SLEDAI-2K total score of 9.9, mean BILAG-2004 total score of 18.1, mean PGA of 59.7 and mean prednisone or equivalent daily dose of 11.1 mg (table 2). A total of 79.3% have received concomitant antimalarials and 64.5% immunosuppressive therapy. Baseline levels of circulating lymphocytes, cytokines and bone turnover markers are provided in table 3.
Baseline demographics and patient characteristics
Baseline circulating lymphocyte, cytokine and bone turnover markers (mITT population)
To gain a better understanding of the relationship between our measures of efficacy, we computed correlation coefficients between them. Statistically significant correlations were found when comparing total SLEDAI-2K total score with the BILAG-2004 total score (r=0.4, p<0.0001) and CLASI total activity score (r=0.4, p<0.0001); PGA with tender and swollen joint counts (r=0.4, p<0.0001), CLASI total activity score (r=0.2, p=0.0032) and the BILAG-2004 score (r=0.2, p=0.0108); and the BILAG-2004 total score with the CLASI total activity score (r=0.5, p<0.0001) and tender and swollen joint counts (r=0.2, p=0.0026).
Discussion
In this large, randomised, double-blind, placebo-controlled study of RCI for the treatment of persistently active SLE, demographics and baseline characteristics are consistent with those from other studies of active SLE populations.18 25 Activity of SLE in this cohort is indicated by a mean SLEDAI-2K total score of 9.9, mean BILAG-2004 total score of 18.1 and mean PGA of 59.7. Most of the currently enroled patients (95.3%) are on a prednisone (or equivalent) dosage of ≤20 mg/day.
This trial will use the validated composite response measure SRI-4 as the primary endpoint and will also evaluate pharmacodynamics (cell surface markers for lymphocytes, cytokines and serum bone turnover markers).
Biological activity of RCI in the treatment of SLE may extend beyond stimulation of adrenal corticosteroid production. MC1R, MC3R, MC4R and MC5R are expressed on multiple leucocyte subpopulations (eg, T and B cells, macrophages), as well as within target organs (eg, skin, kidney, central nervous system) relevant to SLE.13 In a murine model, RCI has been shown to reduce B-cell differentiation and development, and to decrease circulating autoantibodies, proteinuria, renal lymphocyte infiltration and glomerular immune complex deposition.11 Finally, in an in vitro study using peripheral blood B cells isolated from healthy human subjects, RCI dose dependently inhibited IL-4/CD40 ligand-induced B-cell proliferation and IgG production without enhancing cell death.26
These data suggest that RCI not only directly affects human B-cell function but also provides supportive evidence for steroid-independent effects of RCI when used as a treatment for SLE. Therefore, it is hypothesised that, in addition to clinical improvements, the steroid-independent effects of RCI will result in the reduction of inflammatory cytokines and minimal bone turnover rates.
Potential limitations of this study are the lack of stratification by factors beyond location and prednisone (or equivalent) dose. Factors such as disease severity and/or background therapies could affect the response to RCI therapy.
Conclusions
This randomised, double-blind, placebo-controlled study was designed on the basis of signals of efficacy and safety observed in a previous pilot study. The findings are intended to support RCI as an effective therapeutic option for the treatment of patients with persistently active SLE receiving moderate-dose corticosteroids. Correlations between clinical outcomes and pharmacodynamic measures, including circulating lymphocyte profiles, cytokines and bone turnover markers may help elucidate the mechanisms of RCI.
Acknowledgments
This manuscript is based on work previously presented at the Annual European Congress of Rheumatology (EULAR 2020) and published as a conference abstract. Professional writing and editorial support were provided by MedLogix Communications, Itasca, Illinois, USA, under the direction of the authors.
References
Footnotes
Contributors All authors revised this manuscript critically for intellectual content and approved the final version to be published.
Funding This study was funded by Mallinckrodt Pharmaceuticals.
Competing interests EZ, JZ and EC-S are the current employees and shareholders of Mallinckrodt Pharmaceuticals.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication Not required.
Ethics approval This study was approved by the Western Institutional Review Board centrally and by the local ethics committees/institutional review boards at individual study sites.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement No data are available.