Article Text
Abstract
Objective Antidouble-stranded DNA (dsDNA) antibodies are essential for diagnosis and follow-up of systemic lupus erythematous (SLE). To ensure the best diagnostic approach, most healthcare laboratories opt for a combination of highly sensitive methods, such as solid-phase immunoassays, and highly specific methods, such as the Crithidia luciliae indirect immunofluorescence test (CLIFT). Even so, discordant results are common, thus hindering the diagnostic process. Therefore, this study aimed to characterise a cohort of patients with discrepant results for a dsDNA fluorescence enzyme immunoassay (FEIA) and CLIFT during 2016–2018 and to follow patients up until December 2021.
Methods We performed an observational, longitudinal and retrospective study on 417 samples from 257 patients who had been referred for suspected connective tissue diseases or followed up after diagnosis. All of them were positive for antinuclear antibodies (ANAs) using an indirect immunofluorescence assay (IFA) on Hep-2 cells, the entry criterion in our laboratory, and positive for FEIA dsDNA. Samples were then tested with CLIFT according to our routine protocol, which includes CLIFT testing after FEIA dsDNA results ≥10 UI/ml. After the assessment of data quality, the final analysis was based on 222 patients.
Results Eighty-three patients (37.4%) had positive results in both tests and met the diagnostic criteria for SLE. However, 139 patients (62.6%) had discrepant results (FEIA+, CLIFT–). Of these, 58 patients (41.7%) had a diagnosis of SLE, with 47 (33.8%) having been previously diagnosed and under treatment. The remaining 11 patients (7.9%) had a new diagnosis of SLE, which was made up within 4 years of the initial screening. A total of 81 of the 139 patients (57.5%) with discrepant results did not meet lupus criteria during the follow-up period.
Conclusions The study showed that CLIFT could be negative in both treated and newly diagnosed SLE, thus underlining the importance of follow-up of dsDNA-positive results using solid-phase tests. Therefore, quantitative tests such as FEIA could add value to the diagnosis and management of patients with suspected SLE.
- Systemic Lupus Erythematosus
- Autoantibodies
- Health services research
Data availability statement
Data are available upon reasonable request. All data collected, including fully anonymised participant data, are available. This information includes fully anonymised participant data and a data dictionary. Related documents (study protocol, statistical analysis and ethics committee approval) are available from the date of publication onward at the email address aurora.jurado.sspa@juntadeandalucia.es. Data will be shared after approval of proposals by the Ethics Committee of University Hospital Reina Sofía.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
What is already known on this topic
The Crithidia luciliae indirect immunofluorescence test (CLIFT) has long been regarded as the gold standard in the diagnosis of SLE, but its limited sensitivity has been well documented.
Solid phase assays are more sensitive for measuring double-stranded DNA (dsDNA) antibodies, but they have low specificity, which results in seemingly ‘false positives’.
What this study adds
This study follows patients with discrepancies between dsDNA solid-phase assay and CLIFT results over time.
How this study might affect research, practice or policy
The study provides new evidence that can aid laboratory experts in explaining to rheumatologists the discrepancies between CLIFT and fluorescence enzyme immunoassay results. We provide the following recommendations:
Specifications on the type of antibodies recognised by each method must be specified in the clinicians’ report (avidity, Ig isotype and the method itself).
It is not appropriate to consider dsDNA-positive results that match CLIFT-negative results as false positives; instead, patient follow-up should be advised.
Introduction
Antidouble-stranded DNA (anti-dsDNA) autoantibodies play a pivotal role in the diagnosis and follow-up of SLE1–3 and are related to the development of lupus nephritis.4 5 The 2019 European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR) criteria for classifying SLE include positive values for SLE-specific antibodies, such as anti-dsDNA autoantibodies, as the criterion with the highest weight in the immunology domains. Moreover, anti-dsDNA autoantibodies must be measured using an assay with at least 90% specificity against relevant disease controls.2 In addition, antibody titres are included in the Systemic Lupus Erythematous Disease Activity Index.6 Several anti-dsDNA autoantibody tests are commercially available. However, discrepancies between the results they generate are expected owing to the heterogeneity of the assays (differences in the power of test methods to detect high-avidity or low-avidity antibodies) and of autoantibody populations (differences in the avidity level of dsDNA autoantibodies).3 This heterogeneous landscape generates intense debate and has recently been reviewed.7 In fact, variability between analytical approaches is one of the main barriers to further standardisation of evaluation methods.7–9 Consistent discrepancies are frequently recorded between the Crithidia luciliae indirect immunofluorescence test (CLIFT) and solid-phase tests.10 The Farr radioimmunoassay (Farr-RIA) is considered the gold standard for detecting anti-dsDNA antibodies. However, due to the employ of radioisotopes, the Farr-RIA is no longer used in most laboratories. Instead, CLIFT, solid-phase immunoassays, and—better still—a combination of both techniques is regarded as the best approach in clinical practice. With a specificity ranging from 95.0 % to 100 % in most studies, a positive CLIFT result is considered pathognomonic for SLE.8 However, its sensitivity is very variable, as low as <6%,7 and lower than that of solid-phase tests,8 especially in early stage SLE.11 Moreover, CLIFT is an observer-dependent test and, being semiquantitative, does not adequately determine antibody levels, thus reducing its usefulness for monitoring disease.8 The solid-phase dsDNA assay EliA (Thermo Fisher Scientific, Freiburg, Germany) is a robust fluorescence enzyme immunoassay (FEIA) for the detection of anti-dsDNA autoantibodies that measures IgG antibodies in vitro. The median specificity of this method is 94.2% (91.0%–97.7%), which differs little from that of CLIFT, although its sensitivity is higher (median, 54.5% (26.7%–81.0%)).8 Indeed, none of the available assays used to diagnose SLE is sensitive and specific enough, and harmonisation between the methods is a goal that has not yet been attained.8 9
In our laboratory, we use an algorithm to study ANA-positive patients for whom anti-dsDNA antibody testing is requested. The first step of the algorithm involves a solid-phase dsDNA assay. Positive and borderline samples (FEIA dsDNA result ≥10 UI/ml) are then tested using CLIFT for confirmation. However, given the reduced usefulness of CLIFT for the follow-up of SLE, cases with a previous positive CLIFT result are not retested (figure 1). In our routine, as also reported in the literature,10 discrepancies are sometimes found between these tests, mainly when positive values in the solid phase assay are low or within the equivocal range. In these cases, reporting conflicting results to clinicians is challenging. Therefore, our main objectives were to characterise a cohort of samples displaying discrepant results for FEIA and CLIFT during 2016–2018 and to analyse patient follow-up until December 2021.
Schematic algorithm for detection of anti-dsDNA autoantibodies. First, ANA screening was performed on HEp-2 cells. Positive samples (≥1/160 titre) were analysed using a solid-phase fluorescence enzyme immunoassay (FEIA). When the results were ≥10 IU/mL, the presence of anti-dsDNA autoantibodies was subsequently confirmed by CLIFT (Crithidia luciliae indirect immunofluorescence test). *Cases with a previous positive CLIFT result were not retested. dsDNA, double-stranded DNA.
Methods
Study design and participants
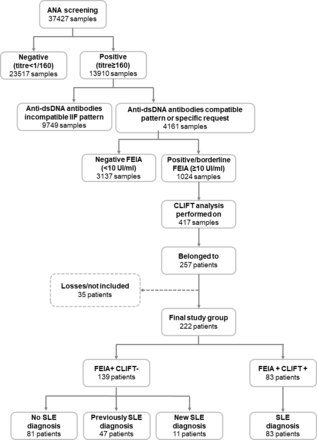
Our retrospective descriptive study initially included all consecutive serum samples referred between 2016 and 2018 to a tertiary hospital for ANA screening (37 427 samples). Of the 13 910 ANA-positive samples, 4161 were analysed using dsDNA FEIA; 1024 samples were borderline (10–15 IU/mL) or positive (≥ 15 IU/mL). According to our routine laboratory testing algorithm (figure 1), not all samples displaying FEIA-positive results are tested using CLIFT. Therefore, only 417 samples from 257 patients were also analysed using CLIFT. There were 35 losses for various reasons (absence of diagnosis in the history, lack of quantitative results for dsDNA FEIA). Data from 222 patients were finally included (figure 2). Samples and patients were classified according to CLIFT and FEIA dsDNA results as double-positive (FEIA+ and CLIFT+) or discrepant (FEIA+ and CLIFT−).
Workflow with the classification of the study group according to the results of the fluorescence enzyme immunoassay (FEIA) and Crithidia luciliae indirect immunofluorescence test (CLIFT) following the laboratory algorithm (see figure 1). Only 417 positive/borderline samples in FEIA were tested with CLIFT, given that results were already available for the remaining samples. dsDNA, double-stranded DNA.
Laboratory assays
The EliA assay was performed using a Phadia 250 System (Phadia AB, Uppsala, Sweden). Details of the complete procedure can be found elsewhere.12 This method provides quantitative information in IU/mL. The variable results were also categorised as negative (<10 IU/mL), equivocal (10–15 IU/mL) and positive (>15 IU/mL), according to the manufacturer’s instructions.
The CLIFT assay and ANA testing on Hep-2 cells were performed on Biosystems slides (Biosystems, Barcelona, Spain) at 1/10 and 1/160 standard titrations, respectively, as previously described for each method.13–15 After a manual reading, the CLIFT result was reported qualitatively and the ANA result as titration levels (titre). The International Consensus on ANA Patterns (ICAP) nomenclature was used to assign the type of ANA pattern.16
Data collection
In the case of patients with discrepant FEIA/CLIFT samples, laboratory variables (ANA titre, Anti-cell 1 (AC-1) ANA pattern, FEIA, CLIFT) and clinical variables (2019 EULAR criteria, age, sex) were extracted from medical records and analysed extensively up to December 2021 for a possible confirmed diagnosis of SLE during the 2016–2021 follow-up. Diagnosis of SLE was based on the physician’s global assessment according to the 2016 and, subsequently, 2019 EULAR/ACR criteria.2
This retrospective observational study was conducted according to national regulations and institutional policies and the tenets of the Declaration of Helsinki. Patient data were anonymised according to the Spanish official procedure.17
Statistical analysis
The Kolmogorov-Smirnov test was used to assess the normality of data distribution. Descriptive statistics were reported as the mean, median, SD and IQR for continuous variables (age, FEIA, ANA titre) and as frequencies and percentages for discrete variables (sex, AC-1 ANA pattern, CLIFT, categorised FEIA, EULAR 2019 criteria).
Differences in continuous variables between the two groups were assessed using the t test for those variables that were normally distributed according to the Shapiro–Wilk test, with equal or unequal variances selected depending on the result of the Levene test; the analysis of variance test was used for comparison between more than two groups. Non-normally distributed continuous variables were assessed using the Mann-Whitney test (two groups) or the Kruskal-Wallis test (more than two groups). Pearson’s χ2 test was used to assess differences between discrete variables. Statistical significance was set at p<0.05 with 95% CI.
Availability of data and materials
All data collected, including fully anonymised participant data, are available. This information includes fully anonymised participant data and a data dictionary. Related documents (study protocol, statistical analysis and ethics committee approval) are available from the date of publication onwards at the email address aurora.jurado.sspa@juntadeandalucia.es.
Data will be shared after approval of proposals by the Ethics Committee of University Hospital Reina Sofía.
Results
According to the results of CLIFT and the final diagnosis, we identified four patient groups (table 1). Eighty-three patients were CLIFT-positive, and 139 were CLIFT-negative. In the CLIFT negative group, 81 patients did not meet the EULAR 2019 criteria for SLE, 11 patients met the EULAR 2019 criteria and were diagnosed during the study period, and 47 patients fulfilled the EULAR 2019 criteria having been previously diagnosed and were under treatment (figure 2). Mean age was 49 years (SD 19), and most patients were women (n=188, 84.7%). The median ANA titre was 1/320 (IQR 1/160–1/640). The median FEIA value was 24 IU/mL (IQR 15.7–50.5). The AC-1 (homogeneous) pattern in the ANA test was recorded in 129 cases (64.2%). The demographic, clinical and laboratory data of the whole population and each group are shown in table 1.
Baseline characteristics of the study population
Additionally, comparisons were made between the groups of interest, as follows: (A) CLIFT-negative and CLIFT-positive; (B) The three CLIFT-negative groups; (C) CLIFT-negative without SLE and CLIFT-negative with newly diagnosed SLE; (D) CLIFT-negative with newly diagnosed SLE and CLIFT-positive; (E) The four groups with each other (table 2). Regarding CLIFT status (comparison A), CLIFT-positive patients were significantly younger (p=0.005), had higher ANA titres (p<0.001) and higher solid-phase assay dsDNA values (p<0.001), and presented mainly the ICAP AC-1 pattern (p<0.001). Both groups were predominantly female, with no differences between them. The exact significance profile was obtained when the variables were compared in the four study groups (comparison E) (table 2). We did not find differences when comparing the main characteristics between the three CLIFT-negative subsets (comparison B). Moreover, we could not find any differences when we compared these variables for the CLIFT-negative group without SLE and newly diagnosed SLE (comparison C). Finally, when CLIFT-negative de novo SLE was compared with the CLIFT-positive group, only age was significantly lower in the latter (comparison D) (table 2).
Comparison of variables between the study groups
When the results for FEIA were categorised, the values for the CLIFT-positive patients showed significantly higher values than the other three groups (p<0.001) (table 1). Among the CLIFT-negative patients who were newly diagnosed with SLE during or after the inclusion period, three showed values of 10–15 IU/mL, which were considered grey-zone values, and one of them was diagnosed 4 years after the test. Furthermore, solid-phase assay values below 50 IU/mL were detected in 37 patients (78.7% of CLIFT-negative patients previously diagnosed with SLE). Finally, among double-positive patients, only 11 (13.25%) had grey-zone values.
Discussion
This study showed that solid-phase assays can increase sensitivity in diagnosis of SLE. dsDNA FEIA identified 58 CLIFT-negative patients as SLE cases. Even more critically, 11 patients in this group had a new SLE diagnosis; 4 were diagnosed between 1 year and 4 years after the initial test. However, there were also 81 patients with an EliA dsDNA-positive result, who did not develop SLE during the follow-up period. We compared the demographic and laboratory data between the groups. With these comparisons, we tried to find some singularities that would help us predict which patients with a CLIFT-negative result would progress to SLE. Compared with the double-positive SLE population, patients from the CLIFT-negative group with newly diagnosed SLE were slightly older and had a lower antibody titre, lower frequency of the AC-1 pattern and lower dsDNA FEIA values. In addition, the percentage of men was higher than expected in patients with SLE. However, these differences were only marginally significant for the variable age (comparison D; table 1). This group might represent patients with SLE in whom the fundamental clinical and analytical spectrum that characterises active SLE has yet to develop. However, the older age and lower female predominance in this group would not support this interpretation. Alternatively, it has been reported that positive CLIFT results could identify a group of patients with SLE with specific features.18 In fact, comparison of the characteristics of CLIFT-positive and CLIFT-negative patients (with or without SLE) (comparison A, table 2) revealed that positive CLIFT results were associated with higher dsDNA FEIA values and the homogeneous pattern, as previously described.19 Highly significant differences were also detected for the remaining variables, except for sex. Similarly, a comparison of the variables between the four groups showed the same pattern (comparison E, table 2).
It is essential to characterise CLIFT-negative patients newly diagnosed with SLE if we are to identify biomarkers that could help us to predict the progression towards the disease. In this respect, it is puzzling that the demographic and clinical features of CLIFT-negative patients with newly diagnosed SLE (age, sex, ANA titre, ANA pattern and solid-phase assay) did not differ from those of the remaining CLIFT-negative subsets (comparison B; table 2). Similarly, explicit comparison between CLIFT-negative patients without SLE and CLIFT-negative patients with newly diagnosed SLE revealed no differences, either statistically or as a trend (comparison C; table 2). Although the newly diagnosed SLE group was small, this behaviour reinforces the need to monitor patients with a positive solid-phase dsDNA assay result, as recently reviewed.20 21
Also noteworthy is the predominance of women in the whole cohort (84.7%) and in all subsets, regardless of their final diagnosis. It is well known that autoimmune diseases in general and SLE, predominantly affect women.22–26 However, the inclusion criterion in our cohort of patients was not a specific disease but positive dsDNA findings in a solid-phase assay. This issue again underlines the need for surveillance of this patient group. On the opposite side, it would be necessary to clarify that those patients who ultimately fulfilled classification criteria in the immunoassay-positive population did not rely on this to fulfil the requirements.
The positivity of the EliA dsDNA FEIA ranges from ≥10 IU/mL to >50 IU/mL. Grey-zone values were defined as lower levels of positivity (10–15 IU/mL). This aspect merits special attention, because three patients diagnosed years after the initial screening had levels within the grey zone. In addition, dsDNA FEIA results were below 50 IU/mL in more than 78% of CLIFT-negative patients with previously diagnosed SLE. This low autoantibody level could be influenced by treatment of SLE, which can reduce antibody titres. Moreover, this result might indicate that CLIFT displays negative results after treatment faster than FEIA, probably owing to its lower sensitivity, as previously reported.12 27 Furthermore, CLIFT only detects specific high-avidity dsDNA autoantibodies, while FEIA detects mainly high-avidity and intermediate-avidity dsDNA autoantibodies.8 In the group of patients with positive results in both tests and a diagnosis of SLE, 13% had levels within the grey zone. Therefore, although FEIA specificity decreases by the low cut-off value, it is relevant to highlight that while dsDNA is low, it is present in many patients with SLE. Eleven patients (14.8%) are at the opposite side of the spectrum, with solid-phase dsDNA values ≥50 IU/mL without SLE after the follow-up period, underlining once again the need for long-term surveillance of these patients.20
The main limitation of this study is its retrospective design, which is based on actual data from clinical practice. Therefore, there are notable losses of specific patient groups of interest. Indeed, we must consider the loss of patients with a positive CLIFT result before the study period. According to our centre’s work algorithm, these patients routinely undergo solid-phase dsDNA testing but do not undergo a repeat CLIFT test. Moreover, to calculate the diagnostic performance of both tests, it would be necessary to include patients with a negative dsDNA solid-phase result and, possibly, CLIFT-negative or CLIFT-positive patients. Such data have already been extensively published.8 28 In any case, the focus of our work was to study the discrepancies that challenge us in daily practice, mainly solid-phase positivity versus CLIFT negativity. Additionally, the samples selected in the study were not initially categorised according to suspected diagnosis or stage of disease because this information is rarely available on request to the laboratory. Clinical information was obtained after reviewing medical records. Probably a separate analysis would have shown different clinical implications of the discordant results, especially if patients are under treatment.
Regarding the generalisation of the results, according to the workflow shown in figure 2, the selection of cases to be studied was based on a representative sample that included all consecutive cases received in the laboratory for ANA testing over 3 years and subsequent follow-up ranging from 3 years to 6 years. However, since this study was carried out in a single centre in a specific geographical area (southern Spain), it would be difficult to extrapolate our results outside our setting. Nevertheless, the values for the differences in the variables between the groups were obtained with an explicitly stated 95% CI.
The main strength of the present study is that, to our knowledge, it is the first to clinically characterise and follow-up patients with discrepant results in a dsDNA solid-phase assay and CLIFT. Thus, our findings could help laboratory specialists when reporting results to the clinician. While a considerable percentage (33.8%) corresponds to previously diagnosed SLE, 7.9% of CLIFT-negative patients progressed to SLE during the follow-up period. Consequently, CLIFT-negative results do not enable us to rule out SLE.
The diagnostic validity of anti-dsDNA FEIA has been recently revised in an article by analysing the sensitivity and specificity results of several studies and meta-analyses. The median sensitivity was 54.5% (range 26.7%–81%) and the median specificity was 94.2% (range 91%–97.7%),8 which is above the 90% recommended in the 2019 EULAR/ACR criteria for diagnosis of SLE.2 The positive likelihood ratio, calculated from these specificity and sensitivity values, is 13.5, which is considered highly relevant.29 Positive and negative likelihood ratios for a given test are a more harmonised way of reporting the diagnostic performance of this test.30 31
On the other hand, it has been suggested that dsDNA FEIA could eventually replace CLIFT for diagnosis of SLE;32 nevertheless, it is important to take account of possible false-positive results, mainly in the low positive range.33 Indeed, it should be noted that in our study 58% of patients with a negative CLIFT result did not develop SLE during the follow-up period.
In summary, patients with positive solid-phase dsDNA autoantibody values should be followed up in the long term, even when their CLIFT result is negative, and they do not fulfil ACR/EULAR criteria. Thus, we will be able to improve early detection of SLE, which will result in early treatment and improved quality of life for our patients. In addition, a multicentre longitudinal study is needed to characterise CLIFT-negative patients who develop SLE to identify biomarkers that can help us to predict disease progression.
Data availability statement
Data are available upon reasonable request. All data collected, including fully anonymised participant data, are available. This information includes fully anonymised participant data and a data dictionary. Related documents (study protocol, statistical analysis and ethics committee approval) are available from the date of publication onward at the email address aurora.jurado.sspa@juntadeandalucia.es. Data will be shared after approval of proposals by the Ethics Committee of University Hospital Reina Sofía.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants. This retrospective observational study was conducted according to national regulations and institutional policies and the tenets of the Declaration of Helsinki. It was approved by the Ethics Committee of University Hospital Reina Sofía (approval number 5122). Patient data were anonymised according to the Spanish official procedure. No samples were analysed. The study just recovered anonymised results from routine testing.
Acknowledgments
We used the STROBE cohort checklist when writing our report. The authors thank Mercè Tena from Thermo Fisher Scientific for her writing support. The authors thank Content ed Net Madrid for editorial support and Thermo Fisher Spain for funding.
References
Footnotes
Contributors AJR conceived and designed the study. ATA and RBS selected the study cohort. ATA reviewed the histories and performed the lupus classification according to the EULAR/ACR 2019 criteria. ATA, RBS, JAM, AN and PAR collected demographic, clinical and laboratory data and developed the database. AJR and AN performed the statistical analyses. AJR, ATA and AN wrote the draft. AJR is responsible for the overall content as guarantor and accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish. All the authors reviewed the article for important intellectual content and approved the submitted version of the manuscript. Moreover, all the authors have agreed both to be personally accountable for the authors' contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests The last author (AJR) has participated as a speaker in three scientific conferences sponsored by Thermo Fisher Scientific.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.