Article Text
Abstract
Objectives Commercial curcumin (CU), derived from food spice turmeric (TU), has been widely studied as a potential therapeutic for a variety of oncological and inflammatory conditions. Lack of solubility/bioavailability has hindered curcumin's therapeutic efficacy in human diseases. We have solubilised curcumin in water applying heat/pressure, obtaining up to 35-fold increase in solubility (ultrasoluble curcumin (UsC)). We hypothesised that UsC or ultrasoluble turmeric (UsT) will ameliorate systemic lupus erythematosus (SLE) and Sjögren's syndrome (SS)-like disease in MRL-lpr/lpr mice.
Methods Eighteen female MRL-lpr/lpr (6 weeks old) and 18 female MRL-MpJ mice (6 weeks old) were used. Female MRL-lpr/lpr mice develop lupus-like disease at the 10th week and die at an average age of 17 weeks. MRL-MpJ mice develop lupus-like disease around 47 weeks and typically die at 73 weeks. Six mice of each strain received autoclaved water only (lpr-water or MpJ-water group), UsC (lpr-CU or MpJ-CU group) or UsT (lpr-TU or MpJ-TU group) in the water bottle.
Results UsC or UsT ameliorates SLE in the MRL-lpr/lpr mice by significantly reducing lymphoproliferation, proteinuria, lesions (tail) and autoantibodies. lpr-CU group had a 20% survival advantage over lpr-water group. However, lpr-TU group lived an average of 16 days shorter than lpr-water group due to complications unrelated to lupus-like illness. CU/TU treatment inhibited lymphadenopathy significantly compared with lpr-water group (p=0.03 and p=0.02, respectively) by induction of apoptosis. Average lymph node weights were 2606±1147, 742±331 and 385±68 mg, respectively, for lpr-water, lpr-CU and lpr-TU mice. Transferase dUTP nick end labelling assay showed that lymphocytes in lymph nodes of lpr-CU and lpr-TU mice underwent apoptosis. Significantly reduced cellular infiltration of the salivary glands in the lpr-TU group compared with the lpr-water group, and a trend towards reduced kidney damage was observed in the lpr-CU and lpr-TU groups.
Conclusions These studies show that UsC/UsT could prove useful as a therapeutic intervention in SLE/SS.
- Autoantibodies
- Autoimmunity
- Sjøgren's Syndrome
- Systemic Lupus Erythematosus
- Treatment
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
This is the first study to use heat/pressure solubilized curcumin and turmeric.
Curcumin/turmeric supplied in lieu of water ad libitum to MRL-lpr/lpr mice brought about delayed onset of autoantibodies, proteinuria as well as significant decrease in salivary gland infiltration and drastically reduced lymphadenopathy compared to mice supplied water.
Curcumin increased mice survival and turmeric (at 5 mg/mL water) was toxic in MRL-lpr/lpr but not in control MRL-MpJ mice.
Introduction
Systemic lupus erythematosus (SLE) is a devastating, chronic and inflammatory autoimmune disorder that can virtually affect any organ in the body. SLE occurs 10 times more commonly in women than men, and autoantibodies are a cardinal feature of the disease, occurring up to 10 years prior to disease diagnosis.1–5
Sjögren's syndrome (SS) shares some of the features of SLE, being a debilitating systemic and chronic inflammatory, autoimmune disorder characterised by autoantibodies. The main features of SS are diminished lacrimal/salivary gland secretion (sicca complex), resulting in keratoconjunctivitis sicca and xerostomia.6–8
MRL-lpr/lpr mice as an animal model of SLE/SS
MRL-lpr/lpr mice resemble human SLE in several ways, and therefore, is a widely used animal model.9–18 MRL-lpr/lpr mouse has a single-gene mutation (lpr) of the fas apoptosis gene on mouse chromosome 19, and therefore, a defect in apoptotic death of lymphocytes. There is massive accumulation of CD4−CD8− T lymphocytes and development of a disease similar to human SLE. Even though lymphoproliferation in human lupus is not as prominent in majority, other features like lupus nephritis, skin lesions, arthritis and neurological manifestations match well. However, lymphadenopathy with double-negative T-cell proliferation may mimic the defective apoptotic mechanisms and its clearance in human lupus.
MRL-lpr/lpr mice develop salivary and lacrimal gland lymphocytic infiltrates similar to human SS, but without functional loss.13–15
MRL-MPJ mice
MRL/MpJ mice, even though they have the normal Fas gene, develop autoimmune symptoms much later than the MRL-lpr/lpr mice. MRL/MpJ inbred female mice have a lifespan of 73 weeks, while males live for 93 weeks. MRL-lpr/lpr female mice typically die at 17 weeks of age while the males die at 22 weeks. MRL-MpJ mice serve as a control for the MRL-lpr/lpr mice (http://jaxmice.jax.org/strain/000486.html).
The aetiology of SLE/SS is largely unknown. There is no effective treatment for SLE/SS, other than immunosuppressives. Current treatment modalities are fraught with significant side effects. Therefore, it is paramount to have studies that can identify new therapeutic agents with few side effects.
Ultrasoluble curcumin
Our studies showing significantly increased oxidative damage in lupus patients19–21 led us to investigate an antioxidant/anti-inflammatory agent known as curcumin,22–31 obtained from the rhizome of Curcuma longa (turmeric). Curcumin (1,7-bis[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione), a naturally occurring ‘nutraceutical’, comprises 4–6% of turmeric.
Commercial curcumin contains curcumin compound (77%), demethoxycurcumin (DMC) (17%) and bisdemethoxycurcumin (BDMC) (3%). Curcumin, when abbreviated CU, will refer to the commercially available mixture containing all three curcuminoid compounds.
CU has been studied in prostate, oesophagus, lung, breast and oral cancers.32–35 Recently, CU was shown to suppress nuclear factor-kB and increase apoptosis in both Flo-1 and OE33 oesophageal adenocarcinoma cell lines36 ,37 CU also induces release of cytochrome C, activation of caspases and p53 and brings about antiangiogenic effects through downregulation of vascular endothelial growth factor.38 ,39 TU oil has been shown to have antifungal, mosquitocidal, insecticidal, antibacterial, antioxidant, antimutagenic and anticancer activities.40–43
Most in vitro or in vivo studies are carried out with CU dissolved in organic solvents or as CU powder mixed in animal feed. We have shown that heat treatment (100°C) increased CU/TU solubility 12-fold/3-fold, respectively, in water.44 Subsequently, we found that heat/pressure (121°C/15 psi) treatment increased CU solubility 35-fold (ultrasoluble CU (UsC); unpublished data). Our antibody-antigen inhibition studies with CU45 suggested a possible route of therapeutic intervention for experimental SLE and SS. To test this, we administered water, UsC or ultrasoluble turmeric (UsT) to six MRL-lpr/lpr or MpJ mice each.
Materials and methods
Materials
Curcumin was purchased from Sigma Chemical Co., St Louis, Missouri, USA. Turmeric was bought from a local grocery store. Autoantigens Sm and ribonucleoprotein (RNP) were from Immunovision, AK. HEp-2 antinuclear antibody (ANA) assay kit and Crithidia luciliae anti-double-stranded DNA (anti-dsDNA) assay kit were from INOVA Diagnostics, San Diego, California, USA. Accustrip URS 10 Urine Reagent Strips were from Jant Pharmacal Corporation, Encino, California, USA. Secondary antibody conjugates were from Jackson Laboratories, Bar Harbor, Maine, USA. All other chemicals were of reagent grade.
Animals
MRL-lpr/lpr and MRL-MpJ mice were purchased from Jackson Laboratories (Bar Harbor, Massachusetts, USA). Animals were housed in the OMRF Laboratory Animal Resource Center of the Oklahoma Medical Research Foundation with a 12 h light/dark cycle. Mice were fed standard γ-irradiated mouse chow (Picolab Rodent Diet 20, LabDiet, St. Louis, Missouri, USA) and water ad libitum. For mice selected for curcumin or turmeric treatment, heat/pressure solubilised curcumin or turmeric was supplied in the water bottle. All experimental designs were approved by the OMRF Institutional Animal Care and Use Committee according to established guidelines.
Curcumin and turmeric solubilisation
CU and TU were heat-solubilised/pressure-solubilised at 5 mg/mL water (UsC and UsT) and supplied to mice in water bottle. CU, at 5 mg/mL ultrapure water, was heated to boiling in an aluminium foil-covered conical flask on a hot plate for 1 h and autoclaved for 30 min. The heat-solubilised/pressure-solubilised CU was filtered with Whatman #1 filter after cooling. This filtrate (UsC) was supplied to mice in the water bottle. TU, at 5 mg/mL ultrapure water, was heated to boiling on a hot plate for 1 h and centrifuged at 5000 g for 20 min after cooling. The supernatant (UsT) was provided to the mice in the water bottle. We did not autoclave the heated TU since there was no significant difference in TU solubilisation upon autoclaving after heating on hot plate. Ultrapure water was autoclaved and cooled, to be supplied in the water bottle to control group of mice.
Curcumin in vitro uptake studies
For UsC uptake studies, we took advantage of the fact that CU fluoresces at 458 nm with an emission spectra of 505–575 nm.46 For this experiment, 350 µL of Epstein–Barr virus (EBV) transformed human peripheral blood mononuclear cells (PBMCs) (200 000 cells/mL) were added to one chamber of a Lab-Tek 8-chamber tissue culture slide. The chamber slide was placed on the stage insert of a Zeiss LSM 710 Confocal Microscope. The cells were viewed and settings arranged prior to addition of UsC. UsC (350 µL) was added to EBV transformed cells. Immediately after addition of UsC the cells were viewed and photographed every five seconds. Control cells treated with 350 µL of heat-treated water did not show any fluorescence (data not shown).
Experimental treatment
Six-week-old female MRL-lpr/lpr and MRL-MpJ mice were used in the experiment. MRL-lpr/lpr female mice develop lupus-like disease at the 10th week and die at an average age of 17 weeks. Female MRL-MpJ mice get lupus-like disease very late in life (47th week) and typically die at 73 weeks. MRL-lpr/lpr and MRL-MpJ mice were assigned to six groups, with six animals in each group (three animals/cage) as follows. Autoclaved water, UsC or UsT were provided to the animals in the drinking bottle.
Group 1 (lpr-water)—given autoclaved water
Group 2 (lpr-CU)—given UsC
Group 3 (lpr-TU)—given UsT
Group 4 (MpJ-water)—given autoclaved water
Group 5 (MpJ-CU)—given UsC
Group 6 (MpJ-TU)—given UsT
Water, UsC or UsT in the drinking bottles was replenished every three days and volume consumed by each group calculated weekly. Blood and urine was collected biweekly and weekly, respectively.47 ,48 Proteinuria was determined semi-quantitatively by dipstick analysis (Accustrip URS 10) from week 7. MRL-MpJ mice were sacrificed at the end of 36 weeks. MRL- lpr/lpr mice died by 32 weeks of age due to disease.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
Protein in the urine of the mice was also assessed by means of sodium dodecyl sulfate polyacrylamide gel electrophoresis. Aliquots of mouse urine, after removal of cells and casts, were analysed on a 4–20% gradient gel and stained with Coomassie brilliant blue.49 ,50
Sm and RNP ELISA
Sera from serial bleeds of the various experimental groups were analysed for the presence of antibodies against Sm or RNP autoantigens. For this purpose, a direct ELISA using Sm or RNP autoantigen as the solid-phase antigen was carried out as described.47 ,51 Essentially, bovine Sm or RNP was coated on 96-well microtiter plates overnight at 4°C at 5 µg/mL in coating buffer. The plates were blocked with 3% non-fat dry milk in phosphate buffered saline (PBS) for 2 h at room temperature. Primary sera were added and incubated for 2 h at room temperature. After washing four times with PBS containing 0.05% Tween-20 (PBST), appropriate alkaline phosphatase conjugate was added and incubated for another 2 h at room temperature. Following washing four times with PBST, substrate was added and absorbance read at 405 nm.
Indirect immunofluorescence
HEp-2 slides were used according to manufacturer's instructions for determination of ANA by indirect immunofluorescence (IIF) assay. Sera were diluted with PBS (1:40, 1:80, 1:160 or 1:320). The diluted samples were added to the slide, incubated for about 40 min and washed with PBS. The slides were then incubated with mouse FITC conjugate diluted 1:100 with PBS. The slides were washed and viewed using fluorescence microscopy.
C. luciliae assay
C. luciliae slides were used according to manufacturer's instructions for determination of anti-dsDNA antibodies by IIF assay. Briefly, sera were diluted with PBS (1:10, 1:100, 1:200, 1:300 or 1:500). The diluted samples were added to the slide, incubated for about 40 min and washed with PBS. The slides were then incubated with mouse FITC conjugate diluted 1:100 with PBS. The slides were washed and viewed using fluorescence microscopy.
Determination of urinary cell casts
Fresh mouse urine was spun at 200 g for 5 min at room temperature and 5 μL of pellet was applied to a microscope slide, covered with a cover slip and examined using light microscopy.
Histopathological studies
Upon animal sacrifice, portions of kidney, salivary gland and spleen were collected in 10% neutral-buffered formalin and stored at room temperature. Fixed tissues were embedded in paraffin, sectioned at 5 μm and stained with either H&E or periodic acid Schiff's reagent (PAS). PAS staining was performed to evaluate the magnitude of glomerulonephritis in the animals from all treatment groups. A portion of the tissues were flash frozen in 50:50 Optimal Cutting Temperature:Tissue Freezing Medium (Triangle Biomedical, Durham, North Carolina, USA) and cryopreserved at −80°C for immunofluorescence staining. Stained slides were evaluated and scored blindly.
Statistical analysis
Values are given as means±SD or SE for each group, as mentioned in the figures. Statistical analyses were done by either Student's t test or Mann–Whitney U test, and the p-value was arrived at to assess the statistical significance of the changes observed. p<0.05 was considered to be significant.
Results
UsC enters cultured cells in 30 s
Uptake of UsC into B cells occurred within 2 min at room temperature and within 30 s (at 37°C, 5% CO2 within microscope chamber) (figure 1a, c; see online supplementary video). Cells fluoresce only when CU uptake happens. Media containing UsC does not fluoresce. MCF7 cells take up CU (dissolved in dimethylsulfoxide (DMSO)) slowly (takes 30 min to enter cells, and 4 h to saturate cells).46
Lymphadenopathy (at week 14) in the lpr-water, lpr-CU and lpr-TU groups of mice and ultrasoluble curcumin (UsC) uptake by Epstein–Barr virus (EBV) transformed human PBMCs. (a, A–C) EBV-transformed human PBMCs incubated with UsC for 1 min; (a, D–F) EBV-transformed human PBMCs incubated with UsC for 2 min; (a, G–I) EBV-transformed human PBMCs incubated with UsC for 4 min; (A, D, G) fluorescence; (B, E, H) transmitted light overlay; (C, F, I) merged image (fluorescence+transmitted light overlay); (b) lymphadenopathy in the lpr-water group (A–D), lpr-CU group (E–H) and lpr-TU group (I–L). Arrows point to enlarged lymph nodes; (c) enlarged image of UsC uptake by EBV-transformed human PBMCs. (Left panel) fluorescence; (middle panel) transmitted light overlay; (right panel) merged image (fluorescence+transmitted light overlay). CU, curcumin; TU, turmeric.
Efficient absorption and uptake of CU by cells ensures its pharmacological action. Based on these data, we treated groups of MRL-lpr/lpr or MRL- MpJ mice with UsC or UsT to test its efficacy in ameliorating SLE-like or SS-like condition. We allowed the MRL-lpr/lpr mice to come to their prescribed end point, that is, death by disease or undergo sacrifice when death seemed imminent.
Body weights and water, curcumin or turmeric consumption
There was no significant difference in body weights or consumption of water, CU or TU between various groups of mice (table 1A).
Body weights, autoantibodies, lymph node size, glomerular area, volume (water, curcumin and turmeric) consumed per day and histopathology (FS) in submandibular/parotid glands of MRL-lpr/lpr and MRL-MpJ provided water, CU or TU
Treatment with UsC or UsT significantly ameliorates lymphadenopathy in the MRL-lpr/lpr mice
By 14 weeks of age, 5/6 mice in lpr-water group developed significant palpable lymphadenopathy, a major characteristic of the MRL-lpr/lpr mice (figure 1b). Lymphoproliferation was drastically reduced in the lpr-CU and lpr-TU group compared with the lpr-water group (table 1A). The lpr-water group had severe lymphadenopathy (average lymph node (LN) weight of 2606 mg). However, the lpr-TU group had minimal lymphadenopathy (average LN weight of 385 mg) (p<0.02), followed by the lpr-CU group (average LN weight of 743 mg) (p<0.03) (figure 2bA, bB).
Urinary cell casts, lymph node weights and proteinuria in the lpr-water, lpr-CU and lpr-TU groups of mice. (a) Urinary cell casts in the lpr-water group. (A–D) Red or white cell casts in four individual mice belonging to the lpr-water group at week 14. None of the mice in the lpr-CU or lpr-TU group had urinary cell casts at week 14; (bA) average lymph node weights in the lpr-water, lpr-CU and lpr-TU groups of mice. Average lymph node weights: lpr-water group= 2606 mg; lpr-CU= 743 mg; lpr-TU=385 mg. *p=0.03, lpr-water group versus lpr-CU group; **p=0.02, lpr-water group versus lpr-TU group (Mann–Whitney U test). Values are given as means±SE for each group. Statistical analyses were done by Mann–Whitney U test and the p-value was arrived at to assess the statistical significance of the changes observed. p<0.05 was considered to be significant; (bB) Weight of lymph nodes at death for the lpr-water, lpr-CU and lpr-TU groups of mice; (c) proteinuria in the MRL-lpr/lpr and MRL-MpJ mice provided with water, CU or TU analysed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) (third urine collection; week 11). In total, 20 µL urine from each mouse was electrophoresed on 4–20% SDS-PAGE gels and stained with Coomassie brilliant blue. Lanes 1–6: MpJ-water group; lanes 7–12: MpJ-CU group; lanes 13–18: MpJ-TU group; lanes 19–24: lpr-water group; lanes 25–29: lpr-CU group; lanes 31–36: lpr-TU group. Increased protein excretion can be seen in the lpr-water group compared with the lpr-CU, lpr-TU and control MpJ mice. CU, curcumin; TU, turmeric.
Proteinuria and urinary casts in the lpr-water, lpr-CU and lpr-TU groups
The lpr-water group (4/6) developed severe SLE-like disease by week 14, with significant urinary cell casts (figure 2a) and proteinuria (two animals had >2000 mg protein each by dipstick method; two had 300 mg protein each) (figure 2c; table 2). After week 14, the protection by CU or TU from proteinuria greatly decreased.
Proteinuria assessed by the dipstick method (Accustrip URS 10) in the urine of MRL-MpJ and MRL-lpr/lpr mice provided water, CU or TU (third urine collection; week 11)
ANA in the lpr-water, lpr-CU and lpr-TU groups
We determined ANA positivity in various groups using IIF. Sera (1:80 dilution, bleed 3) from 6/6 lpr-water group were positive for ANA (figure 3a, table 1A) (either homogenous or homogenous+nucleolar pattern), weakly positive in the lpr-CU group and negative in lpr-TU mice (our cut-off titre for positivity was 1:40). However, animals from all three groups were positive at 1:80 in the following bleeds, and these sera could be diluted to 1:500 with sustained ANA positivity.
Antinuclear antibody (ANA) patterns (using HEp-2 ANA assay kit) and anti-dsDNA (analysed by Crithidia luciliae assay) in sera of the lpr-water, lpr-CU and lpr-TU groups of mice. (a) ANA patterns observed in sera (third bleed) (1:80 dilution) of the lpr-water group (A–C), lpr-CU group (D–F) and lpr-TU group (G–I); (b) anti-dsDNA observed in sera (first bleed) (1:100 dilution) of the lpr-water group (A–C), lpr-CU group (D–F) and lpr-TU group (G–I). Only results obtained from three animals are shown for each experimental group. CU, curcumin; TU, turmeric.
Anti-dsDNA antibody in the lpr-water, lpr-CU and lpr-TU groups
Anti-dsDNA antibodies were positive (first bleed, 1:100 dilution) in the lpr-water and lpr-CU groups, but not in the lpr-TU groups (figure 3b, table 1A). By the third bleed, the lpr-TU group also developed anti-dsDNA antibodies (data not shown). Anti-dsDNA antibodies remained elevated in all groups in the following bleeds (up to 1:300).
Anti-Sm and anti-RNP in the lpr-water, lpr-CU and lpr-TU groups
There were no significant levels of anti-RNP and anti-Sm autoantibodies in the first bleed (age of eighth week) of the lpr-CU and lpr-TU groups, whereas the lpr-water group had high levels of both anti-RNP and anti-Sm by this time (figure 4aA). However, levels of both antibodies increased by the tenth week (bleed 2) and continued to be elevated. Thus, there is a brief period where antibody levels were lower in the treated groups (lpr-CU and lpr-TU groups). Interestingly MRL-MpJ mice developed anti-RNP and anti-Sm antibodies, albeit lower than lpr groups (figure 4aB).
Anti-RNP/anti-Sm antibodies in sera (bleeds 1–5) and H&E stained sections of kidney/salivary gland of the MRL-lpr/lpr and MRL-MpJ mice provided with water, CU or TU. (aA) Anti-RNP and anti-Sm in sera of the lpr-water, lpr-CU and lpr-TU groups of mice. (aB) Anti-RNP and anti-Sm in sera of the MpJ-water, MpJ-CU and MpJ-TU groups of mice. Values for each bleed were normalised to an anti-Sm or anti-RNP-positive control. Values are given as means±SD for each group. Statistical analyses were done by Student's t test and the p-value was arrived at to assess the statistical significance of the changes observed. p<0.05 or lower was considered to be significant; (b) kidney and salivary gland histopathology. (A) lpr-water, (B) lpr-CU (C) lpr-TU and (D) control MpJ mice. Protein casts in kidney and cellular infiltrates in salivary gland are indicated by arrows. CU, curcumin; RNP, ribonucleoprotein; TU, turmeric.
Histopathological studies in the lpr-water, lpr-CU and lpr-TU groups
The lpr-water group had significant kidney pathology (figure 4b, left panel) and there was a trend towards reduced damage in the lpr-CU and lpr-TU groups (table 1A, figure 4b, left panel). Salivary gland histopathology studies also showed significantly reduced cellular infiltration in the lpr-TU or lpr-CU groups compared with the lpr-water group (table 1B, figure 4b, right panel). The lpr-water group (3/6), for which salivary glands were available for testing, had significant cellular infiltrates (focus score >1). However, none of the four mice in the lpr-TU group, for which salivary glands were available for testing, had significant cellular infiltrates (focus score of 0 or <1), while 4/6 mice in the lpr-CU group had significant cellular infiltrates (table 1B).
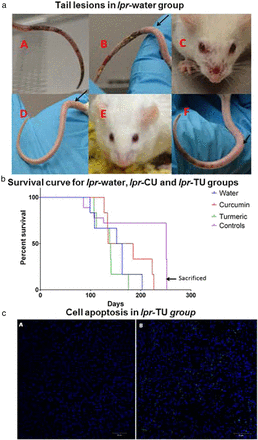
Tail skin lesions occurred in the lpr-water group (figure 5aA, aB) and not in the lpr-CU group (figure 5aD). One mouse from the lpr-TU group had a very mild lesion in the distal end of the tail (week 24) (figure 5aF). The tail necrotic lesions started away from the site of tail bleed incisions and were noted in 2 of the 18 animals subjected to tail bleeding. These two mice were also the sickest with the most aggressive disease manifested by the largest and most numerous LNs in the whole cohort (figure 2bB). Therefore, we attribute this to disease activity rather than to procedural complication. The skin lesions on the face (figure 5aC) were unrelated to barbering.52
Lesions, survival and cell apoptosis in the lpr-water, lpr-CU and lpr-TU groups of mice. (a) Lesions in the lpr-water (A–C), lpr-CU (D and E) and lpr-TU (F) groups. Circumferential erythematous lesions, unrelated to tail vein bleeding, seen in the lpr-water group. Arrows point to cuts made for tail bleeding. (b) Survival curve for the lpr-water, lpr-CU and lpr-TU groups of mice. There was no difference in the survival between the MRL-MpJ mice (used as control mice for the MRL-lpr/lpr mice) fed with water, curcumin or turmeric. The control MpJ mice provided water (MpJ-water), curcumin (MpJ-CU) or turmeric (MpJ-TU) were sacrificed at 260 days. (c) Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) of axillary lymph node section of the lpr-TU group mouse. Apoptotic cells are indicated by dUTP incorporation, shown by FITC labelling. (cA) Overlay of 4′,6-diamidino-2-phenylindole (DAPI) and FITC staining in the absence of terminal deoxynucleotidyl transferase. (cB) Overlay of DAPI and FITC staining in the presence of the enzyme. CU, curcumin; TU, turmeric.
Survival in the lpr-water, lpr-CU and lpr-TU groups
Survival was longer in the lpr-CU group compared with the lpr-water group (figure 5b) (>20% longer lifespan). Two mice belonging to the lpr-water group suffered deaths due to disease during weeks 15 and 16 with enlarged LNs and proteinuria. The first two deaths in the lpr-CU group occurred in the 19th and 20th weeks, but the animals did not have significant lymphadenopathy (figure 2bB). None of the mice in the lpr-CU or lpr-TU groups had urinary cell casts till at least the 15th week.
The lpr-TU group had a shortened lifespan (average 132 days vs 148 days for the lpr-water group) due to complications possibly unrelated to lupus-like illness (table 1A). Five of six mice in the lpr-TU group had intestinal problems (narrowed intestinal lumen, atrophy of stomach, bloody stomach or bloody intestines). Two mice in the lpr-TU group died during weeks 16 and 17 without evidence of lupus, but instead with intestinal complications. Notwithstanding these deaths, none of the lpr-TU group animals had lupus-like illness with minimal evidence of lymphadenopathy.
We hypothesised that the lack of lymphoproliferation could be due to increased apoptosis via a non-fas-mediated pathway. To test this hypothesis, we studied LN of CU-treated or TU-treated animals by using transferase dUTP nick end labelling assay. We detected apoptosis of lymphocytes (figure 5c) in LN of 3/6 mice in the lpr-TU group and 3/6 mice in the lpr-CU group, with no significant apoptosis in the lpr-water group (data not shown). These results explain one possible mechanism for the significantly reduced lymphadenopathy in treated mice.
Discussion
Lack of solubility and bioavailability has hindered CU's therapeutic efficacy in human diseases. Previously reported studies have used CU solubilised in organic solvents or as CU powder supplemented in animal feed. Our earlier studies found that only DMC and BDMC are largely soluble in water at room temperature. However, only when solubilised with heat, curcumin compound becomes soluble.44 Based on these studies, we speculate that for clinical trials carried out with CU given in the powder form as well as animal studies (with the exception of animal studies using CU solubilised in DMSO), the effect seen is a consequence of DMC as well as BDMC and only negligibly from curcumin compound. We hypothesised that UsC or UsT will ameliorate SLE-like/SS-like disease in the MRL-lpr/lpr mice, a well-studied animal model of SLE and SS. As far as we know, this is the first study to investigate the effect of solubilised CU compound, DMC and BDMC in murine lupus/SS.
The major results in our study are (a) a delayed onset of anti-RNP, anti-Sm, ANA and anti-dsDNA in the lpr-CU and lpr-TU groups compared with the lpr-water group; (b) the initial alleviation of proteinuria in the lpr-CU and lpr-TU groups compared with the lpr-water group; (c) drastically reduced lymphadenopathy in the lpr-CU and lpr-TU groups compared with the lpr-water group; (d) increased survival in the lpr-CU group (a 20% survival advantage) compared with the lpr-water group and toxicity of UsT in the MRL-lpr/lpr mice and not in the MRL-MpJ mice; and (e) reduced salivary gland infiltrates in the lpr-CU and lpr-TU groups compared with the lpr-water group.
Delayed onset of anti-RNP, anti-Sm, ANA and anti-dsDNA in lpr-CU and lpr-TU groups compared with the lpr-water group
It is not clear as to how CU or TU induces this initial drop in autoantibody levels in the treated mice compared with the lpr-water group (figures 3 and 4a). Defective expression of Fas results in the inability to delete autoreactive T and B cells in the periphery.53–55 These autoreactive T cells survive in MRL/lpr/lpr mice and help B cells specific for self-antigens to produce autoantibodies. We speculate that autoreactive T and B cells briefly experience a conventional initial tolerance and induction of anergy in response to treatment with CU or TU. Oxidative modification of autoantigen may also account for immunogenicity of self-antigens21 ,56 ,57 and the antioxidant property of CU could delay oxidative modification of autoantigens and thus prevent them from acting as neoantigens for a limited period of time.
Delayed onset of proteinuria in the lpr-CU and lpr-TU groups compared with the lpr-water group
Lupus nephritis and irreversible renal injury result from autoantibodies forming immune complexes and depositing in the glomerulus, as well as glomerular inflammation triggered by locally produced chemokines and cytokines.23 We found delayed onset of proteinuria and urinary cell cast formation (figure 2) in treated mice compared with the lpr-water group. We believe that reduced proteinuria and urinary cell casts observed in the early stages of disease in experimental mice is a direct consequence of inhibition of antigen–antibody interaction by CU.45 We found a trend towards improved glomerulonephritis in the lpr-CU and lpr-TU groups, but the improvement was not significant. Reduced glomerular IgG immune complex deposition and renal inflammation was observed in a recent study that provided CU powder supplemented in animal feed to New Zealand Black/White (NZB/W) F1 female mice. How much of this effect was due to curcumin compound and not DMC and BDMC is unknown. Turmeric powder (500 mg capsules), administered to patients with refractory lupus nephritis, was shown to significantly ameliorate proteinuria.58 Thus, there is a precedence for protection by CU/TU against proteinuria and glomerulonephritis.
Drastically reduced lymphadenopathy in the lpr-CU and lpr-TU groups
We observed a dramatic reduction in lymphadenopathy in both lpr-CU and lpr-TU groups compared with the lpr-water group, with lpr-TU having the most decrease (figure 2bA, bB). We found that induction of non-fas apoptosis by CU or TU is a possible mechanism by which lymphadenopathy was reduced in the lpr-CU and lpr-TU groups. However, we do not know the exact mechanism by which CU or TU induces apoptosis in these mice. It is possible CU depletes reduced glutathione, leading to caspase-dependent and caspase-independent apoptosis.40 ,59–61
CU selectively induces apoptosis in cancer cell lines in several ways.38 ,40 ,61–63 Reduced glutathione (GSH) has been demonstrated to play a vital role in sensitising these cell lines to CU.59 ,60 Depletion of GSH further sensitised cells to the effects of CU, and cell death is caused by generation of reactive oxygen species (ROS). Recent studies have shown CU-induced ROS-dependent depletion of GSH, which led to caspase-dependent and caspase-independent apoptosis in L929 cells.64 Studies have reported disease-dependent decrease of intracellular GSH concentration in MRL-lpr/lpr mice60 and decreased blood sulfhydryl or GSH concentrations in T cells of patients with SLE.64
In spite of dramatic protection from lymphoproliferation, all treated mice (lpr-CU and lpr-TU groups) died by the 32nd week (figure 5c). Two mice from the lpr-water group died in the 14th and 15th weeks, and mainly had severe proteinuria. Mice in the lpr-CU group lived on average ∼20% longer than the lpr-water group. The combination of delayed onset of autoantibodies and proteinuria, as well as dramatic decrease in lymphoproliferation, might be the reason for enhanced survival in the lpr-CU group. On the other hand, lpr-TU lived 16 days on average less than the lpr-water group, in spite of having the least lymphadenopathy of the three groups (table 1A). We attribute this to toxicity of TU in the sick group of mice since TU treatment did not cause toxicity problems in control mice (MRL-MpJ mice).
Two mice from the lpr-water group with maximal lymphadenopathy (figure 2bB) had severe tail lesions (figure 5aA, aB). Skin lesions in the MRL-lpr/lpr mice are thought to be caused by vasculitis secondary to deposition of immune complexes.65 None of the CU treated mice have significant skin lesions at the time when the lpr-water group had the lesions, while one TU-treated mice had a milder skin lesion towards the end of the tail (figure 5aF).
Reduced cellular infiltration in salivary gland of the lpr-CU and lpr-TU groups
Treatment with TU significantly reduced cellular infiltrates in salivary glands of the MRL-lpr/lpr mice (figure 4b, right panel). However, we did not carry out saliva collection in the experimental groups since these mice are well known to not have functional loss. This is one of the limitations of using these mice as an animal model of SS.
Other limitations of the study
Having only six animals in each group is a limitation of this study. Another limitation is the fact that CU and TU were subjected to filtration and centrifugation under non-sterile conditions after autoclaving, while the lpr-water group received autoclaved water directly after cooling (without going through non-sterile treatment conditions). We believe that this may have helped in the longevity of the lpr-water group, which lived longer than expected for this lupus-prone strain. The reason we think this is so is the fact that we observed a subsequent group of 10 MRL-lpr/lpr mice maintained at our animal facility on non-autoclaved regular tap water developed massive LNs much earlier (unpublished data) than the animals used in the current experiment. Yet another limitation is that tissues like salivary glands, kidneys and LNs were often collected from the mice at different time points when the animals actually died. So, it was not possible to study histopathology in these animals at the same time point. Finally, there was a delay in collecting these tissues from some of the animals.
Conclusion
Our data show that UsC or UsT (a) are safe in normal mice but TU at 5 mg/ml gave gastrointestinal side effects in lupus mice (b) reduce disease in MLR-lpr/lpr mice and (c) induced lymphocyte apoptosis in these mice. Thus, these results show feasibility of ameliorating lupus-like/SS-like disease in mice with UsC or UsT.
Acknowledgments
The authors thank Julie Maier, OMRF imaging core facility and Beverly Hurt, OMRF Graphics Resource Center, for their excellent technical assistance. They also thank staff of Laboratory Animal Resource Center, OMRF, for the excellent care and maintenance of animals used in the experiments.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online video
Footnotes
Contributors We assure that all authors included on this paper fulfil the criteria of authorship. Conversely we also assure that there is no one else who fulfils the criteria that has been excluded as an author.
Funding This work was supported by grant # ARO53483 from Rheumatic Disease Research Cores Center, Oklahoma, grant # GM104938 from Clinical and Translational Science Award, NIJ and grant # A1082714 from ACE.
Competing interests None declared.
Ethics approval All experimental designs were approved by the OMRF Institutional Animal Care and Use Committee according to established guidelines.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Data not shown in the manuscript will be provided upon request.